Abstract
Purpose
Pneumococcus is one of the most important causes of invasive infection through the childhood period. In January 2008, the Clinical and Laboratory Standards Institute (CLSI) published revised penicillin breakpoints for Streptococcus pneumoniae and penicillin susceptibility rates of S. pneumoniae increased in Korea. This study was performed to determine the probability of oral amoxicillin for the empirical treatment achieving bactericidal exposure against pneumococcus using pharmacodynamics model.
Methods
Twenty-three isolates of pneumococci were subjected to determine minimum inhibitory concentration (MIC) for -lactams and macrolide. For the -lactams, exposure of T >MIC (time that free drug concentrations remain above the β β ƒ MIC) for 50% of the administration interval have determined the probability of target attainment (PTA), and regimens that had a PTA >90% were considered optimal. An analysis was performed by applying MIC of 23 isolates to a 5000-patient Monte Carlo simulation model.
Results
Among 23 isolates from healthy children, 7 (30.4%) isolates were MIC 1.0 g/mL and 19 (82.6%) were MIC 2≤ µ ≤µ≤ µ g/mL for amoxicillin. Amoxicillin 40 mg/kg/day achieved PTA >90% at MIC 1.0 g/mL but PTA decreased to 52% at MIC 2 g/mL, whereas amoxicillin 90 mg/kg/day can predict 97% of PTA at MIC 2 g/mL. Overall, oral amoxicillin 90 mg/µµ kg/day for the empirical treatment against pneumococcus can expect more successful response in Korean children. Conclusion: Considering the resistantce pattern of pneumococci in Korean children, we estimate that oral amoxicillin 90 mg/kg/day will provide a pharmacodynamic advantage for the empirical treatment against pneumococcus. And low dose amoxicillin or macrolide are expected to have higher chance of treatment failure than high dose oral amoxicillin. (Korean J Pediatr Infect Dis 2011;18:109–116)
Go to : 
References
1). Kim KH, Sohn YM, Kang JH, Kim KN, Kim DS, Kim JH, et al. The causative organisms of bacterial meningitis in Korean children, 1986–1995. J Korean Med Sci. 1998; 13:60–4.

2). Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies ofStreptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980; 142:923–33.
3). Austrian R, Howie VM, Ploussard JH. The bacteriology of pneumococcal otitis media. Johns Hopkins Med J. 1977; 141:104–11.
4). Luotonen J. Streptococcus pneumoniae and Haemophilus influenzae in nasal cultures during acute otitis media. Acta Otolaryngol. 1982; 93:295–9.

5). Kamme C, Ageberg M, Lundgren K. Distribution of Diplococcus pneumoniae types in acute otitis media in children and influence of the types on the clinical course in penicillin V therapy. Scand J Infect Dis. 1970; 2:183–90.

6). Gray BM, Converse GM, Dillon HC Jr. Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979; 140:979–83.

7). Watts JL, Shryock TR, Apley M, Bade DJ, Brown SD, Gray JT, et al. Performance standards for antimicrobial susceptibility testing; Eighteenth informational supplement, M100-S18, Wayne, PA: Clinical and Laboratory Standards Institute. 2008.
8). Kim BN, Bae LG, Kim MN, Park SJ, Woo JH, Ryu J, et al. Risk factors for penicillin resistance and mortality in Korean adults with Streptococcus pneumoniae bacteremia. Eur J Clin Microbiol Infect Dis. 2002; 21:35–42.

9). Lee NY, Song JH, Kim S, Peck KR, Ahn KM, Lee SI, et al. Carriage of antibiotic-resistant pneumococci among Asian children: a multinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP). Clin Infect Dis. 2001; 32:1463–9.

10). Farrell DJ, Morrissey I, Bakker S, Felmingham D. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999–2000 study. J Antimicrob Chemother. 2002; 50(Suppl S1):39–47.

11). Schito GC, Felmingham D. Susceptibility of Streptococcus pneumoniae to penicillin, azithromycin and telithromycin (PROTEKT 1999–2003). Int J Antimicrob Agents. 2005; 26:479–85.

12). Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–7.
13). Centers for Disease Control and Prevention (CDC). Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae-United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2008; 57:1353–5.
14). Dagan R, Klugman KP, Craig WA, Baquero F. Evidence to support the rationale that bacterial eradication in respiratory tract infection is an important aim of antimicrobial therapy. J Antimicrob Chemother. 2001; 47:129–40.

15). Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998; 27:10–22.
16). Craig WA, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996; 15:255–9.

17). O'Brien KL, Nohynek H. Report from a WHO working group: standard method for detecting upper respiratory carriage ofStreptococcus pneumoniae. Pediatr Infect Dis J. 2003; 22:133–40.
18). Wikler MA, Cockerill FR, Bush K, Dudley MN, Dlio-poulos GM, Hardy DJ, at al. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement. M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute;2009.
19). Fallon RM, Kuti JL, Doern GV, Girotto JE, Nicolau DP. Pharmacodynamic target attainment of oral beta-lactams for the empiric treatment of acute otitis media in children. Paediatr Drugs. 2008; 10:329–35.
20). Brandileone MC, Di Fabio JL, Vieira VS, Zanella RC, Casagrande ST, Pignatari AC, et al. Geographic distribution of penicillin resistance ofStreptococcus pneumoniae in Brazil: genetic relatedness. Microb Drug Resist. 1998; 4:209–17.
21). Syrjanen RK, Kilpi TM, Kaijalainen TH, Herva EE, Takala AK. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001; 184:451–9.
22). Kim YK, Lee CK. Oropharyngeal Carriage and Antimicrobial Resistance of S. pneumoniae in Children of Seoul. Korean J Pediatr Infect Dis. 1997; 4:218–24.

23). Kim KH, Lee EJ, Whang IT, Ryu KH, Hong YM, Kim GH, et al. Serogroup and antimicrobial resistance of Streptococcus pneumoniae isolated from oropharynx in children attending day care center. J Korean Pediatr Soc. 2002; 45:346–53.
24). Hansman D BM. A resistant pneumococcus. Lancet. 1967; 2:264–5.
26). Kim SH, Song EK, Lee JH, Kim NH, Lee JA, Choi EH, et al. Changes of serotype distribution of Streptococcus pneumoniae isolated from children in Korea over a 15 year-period (1991∼2005). Korean J Pediatr Infect Dis. 2006; 13:89–98.
27). Kim KH, Kim JE, Park SH, Song YH, Ahn JY, Park PW, et al. Impact of Revised Penicillin Breakpoints for Streptococcus pneumoniae (CLSI M100-S18) on the Penicillin Susceptibility Rate. Korean J Clin Microbiol. 2010; 13:68–71.
Go to : 
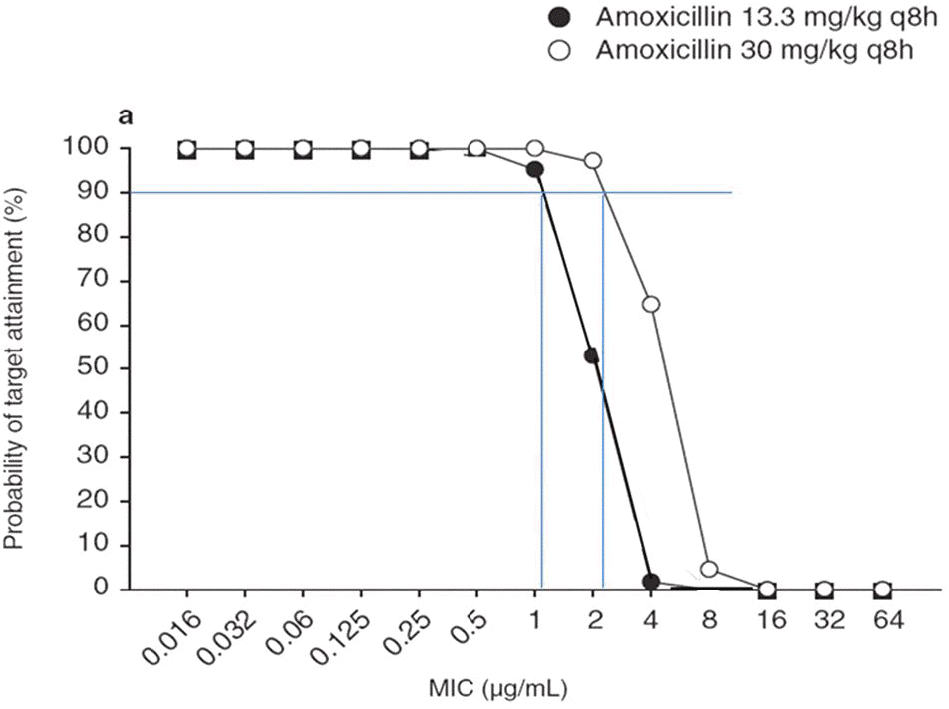 | Fig. 1.Probability of target attainment for amoxicillin-based regimens achieving free drug concentrations above the MIC for 50% of the administration interval 19).(50% T >MIC) at increasing MIC dilutions ƒ |
Table 1.
The Minimum Inhibitory Concentration at which 50% (MIC50) and 90% (MIC90) of Bacteria are Inhibited and the Percent Susceptibility for 23S. pneumoniae Isolates
| Antibiotics | Susceptible MIC* (g/mL) µ | MIC50(g/mL) µ† | MIC90(g/mL) µ† | Percentage susceptible (%) |
|---|---|---|---|---|
| –lactams (nonmeningitis)β | ||||
| Penicillin parenteral | 2≤ | 0.75 | 3 | 78.3 |
| Penicillin oral | 0.06≤ | 0.75 | 3 | |
| Amoxicillin oral | 2≤ | 1.5 | 6 | 82.6 |
| Cefotaxime parenteral | 1≤ | 0.75 | 1.5 | 82.6 |
| Macrolide | ||||
| Erythromycin | 0.25≤ | >256 | >256 | 4.3 |
Table 2.
Distribution of Minimum Inhibitory Concentration of Amoxicillin among 23S. pneumoniae Carriage Isolates Obtained from Healthy Korean Children
| Amoxicillin MIC* (g/mL) µ | No. of isolates indicated | No. of isolates/Total No. (%) |
|---|---|---|
| 1.0≤ | 7 | 30.4 |
| 1< 2.0≤ | 12 | 52.2 |
| 2< 3.0≤ | 1 | 4.3 |
| 3< 6.0≤ | 3 | 13.0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download