Abstract
Purpose
We conducted a prospective comparative clinical study to determine the field efficacy of the 2010-2011 influenza vaccines [Influenza virus strains; A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), B/Brisbane/60/2008] in healthy Korean children under 18 years of age.
Methods
In this study, we enrolled subjects aged between 6 months and 18 years and divided them into 2 study groups: a group who received the influenza vaccines (407 subjects), and a control group who did not receive the influenza vaccines (230 subjects). Ours was a multicenter study that involved 7 hospitals, including the Korea Cancer Center Hospital. The study was conducted between September 2010 and February 2011. We collected nasal wash or throat swab samples from subjects who presented with acute febrile respiratory or influenza-like illnesses at the hospital. We used PCR to confirm the presence of the influenza virus in the respiratory samples and characterize the virus type.
Results
In this study, we collected 22 respiratory samples from the influenza-vaccinated group and found 3 cases of influenza virus infection. Similarly, we collected 21 samples from the control group and found 12 cases of influenza virus infection among 10 subjects during the study period. We determined the field efficacy of the 2010-2011 seasonal influenza vaccines to be 83.2% in healthy Korean children and adolescents.
Figures and Tables
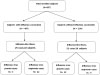 | Fig. 1The flow diagram of the study: comparison of influenza incidence between influenza vaccinated group and unvaccinated group. |
Table 1
Clinical Characteristics and Demographic Features of 637 Subjects Enrolled During 2010-2011 Influenza Season

References
1. Glezen WP. Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL, editors. Influenza viruses. Textbook of pediatric infectious diseases. 2009. 6th ed. Philadelphia: WB Saunders Co;2395–2413.

2. Izurieta HS, Thompson WW, Karamarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000. 342:232–239.

3. Glezen WP, Decker M, Joseph SW, Mercready RG Jr. Acute respiratory disease associated with influenza epidemics in Houston, 1981-1983. J Infect Dis. 1987. 155:1119–1126.

4. Ki HO, Kwon DH. Korean influenza suveillance report, 2010-2011. Public Health Wkly Rep. 2011. 4:1–20.
5. Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating school children against influenza. N Engl J Med. 2001. 344:889–896.

6. Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan, by vaccination of school children. J Infect Dis. 1970. 122:16–25.
8. Hickling J, D'Hondt E. A review of production technologies for influenza virus vaccines, and their suitability for deployment in developing countries for influenza pandemic preparedness. World Health Organization Initiative for Vaccine Research. 2006. 1–34.
9. Fedson DS. Pandemic influenza and the global vaccine supply. Clin Infect Dis. 2003. 36:1552–1561.

10. Fedson DS. Measuring protection: efficacy versus effectiveness. Dev Biol Stand. 1998. 95:195–201.
11. Osterholm MT, Kelly NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systemic review and meta-analysis. Lancet Infect Dis. 2012. 12:36–44.

12. Chang HK, Park JH, Song MS, Oh TK, Kim SY, Kim CJ, et al. Development of multiplex rt-PCR assays for rapid detection and subtyping of influenza type A viruses from clinical specimens. J Microbiol Biotechnol. 2008. 18:1164–1169.
13. Dwyer DE, Smith DW, Catton MG, Barr IG. Laboratory diagnosis of human seasonal and pandemic influenza virus infection. Med J Aust. 2006. 185:S48–S53.

14. Song JY, Cheong HJ, Woo HJ, Wie SH, Lee JS, Chung MH, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011. 26:191–195.

15. Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, et al. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine. 2010. 28:3929–3935.

16. Langley JM, Scheifele DW, Quach C, Vanderkooi OG, Ward B, McNeil S, et al. Safety and immunogenicity of 2010-2011 H1N12009-containing trivalent inactivated influenza vaccine in children 12-59 months of age previously given AS03-adjuvanted H1N12009 pandemic vaccine: A PHAC/CIHR Influenza Research Network (PCIRN) study. Vaccine. 2012. 30:3389–3394.

17. Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practice (ACIP). MMWR Recomm Rep. 2006. 55:1–42.
18. Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA. 2003. 290:1608–1616.
19. Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001. 20:733–740.

20. Gruber WC, Taber LH, Glezen WP, Clover RD, Abell TD, Demmler RW, et al. Live attenuated and inactivated influenza vaccine in school-age children. Am J Dis Child. 1990. 144:595–600.

21. Ruben FL. Prevention and control of influenza. Role of vaccine. Am J Med. 1987. 82:31–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download