Abstract
Purpose
Methods
Results
Conclusion
Figures and Tables
Fig. 1
MFI of GMT against each influenza antigen. Abbreviations : MFI, mean fold increase; GMT, geometric mean titer.

Fig. 2
MFI of GMT against each influenza antigen according to different immune state. (A) MFI of GMT against H1N1 antigen according to ALC count (MFI; ALC≤500/µL: 0.70, ALC >500/µL: 0.94). (B) MFI of GMT against H1N1 antigen according to WBC count (MFI; WBC ≤3,000/µL: 0.66, WBC >3,000/µL: 0.96). (C) MFI of GMT against H3N2 antigen according to ALC count (MFI; ALC ≤500/µL :0.71, ALC >500/µL: 1.30). (D) MFI of GMT against H3N2 antigen according to WBC count (MFI; WBC ≤3,000/µL: 0.87, WBC >3,000/µL: 1.27). (E) MFI of GMT against B antigen according to ALC count (MFI; ALC ≤500/µL: 1.00, ALC >500/µL: 2.09). (F) MFI of GMT against B antigen according to WBC count (MFI; WBC ≤3,000/µL: 1.00, WBC >3,000/µL: 2.14). Abbreviations:MFI, mean fold increase; GMT, geometric mean titer; ALC, absolute lymphocyte count.

Table 1
Patients Characteristics

*two drugs: cisplatin and adriamycin
†three drugs: ifosfamide, adriamycin and etoposide or ifosfamide, adriamycin and dacarbazine
‡recurrence: cyclophosphamide and topotecan
∫ALL protocol: prednisolone, vincristine, daunorubicin, L-asparaginase, cytarabine and methotrexate
∥HDCT: carboplatin, thiotepa and etoposide or ifosfamide, cyclophosphamide and etoposide
Abbreviation: ALL, acute lymphoblastic leukemia
Table 2
Immunogenicity against H1N1
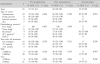
*two drugs : cisplatin and adriamycin
†three drugs : ifosfamide, adriamycin and etoposide or ifosfamide, adriamycin and dacarbazine
‡recurrence : cyclophosphamide and topotecan
∫ALL protocol : prednisolone, vincristine, daunorubicin, L-asparaginase, cytarabine and methotrexate
∥HDCT : carboplatin, thiotepa and etoposide or ifosfamide, cyclophosphamide and etoposide
Abbreviations : ALL, acute lymphoblastic leukemia; HDCT, high dose chemotherapy; ALC, absolute lymphocyte count
Table 3
Immunogenicity against H3N2

*two drugs : cisplatin and adriamycin
†three drugs : ifosfamide, adriamycin and etoposide or ifosfamide, adriamycin and dacarbazine
‡recurrence : cyclophosphamide and topotecan
∫ALL protocol : prednisolone, vincristine, daunorubicin, L-asparaginase, cytarabine and methotrexate
∥HDCT : carboplatin, thiotepa and etoposide or ifosfamide, cyclophosphamide and etoposide
Abbreviations) ALL; acute lymphoblastic leukemia, HDCT; high dose chemotherapy, ALC; absolute lymphocyte count
Table 4
Immunogenicity against B
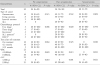
*two drugs : cisplatin and adriamycin
†three drugs : ifosfamide, adriamycin and etoposide or ifosfamide, adriamycin and dacarbazine
‡recurrence : cyclophosphamide and topotecan
∫ALL protocol : prednisolone, vincristine, daunorubicin, L-asparaginase, cytarabine and methotrexate
∥HDCT : carboplatin, thiotepa and etoposide or ifosfamide, cyclophosphamide and etoposide
Abbreviations : ALL, acute lymphoblastic leukemia; HDCT, high dose chemotherapy; ALC; absolute lymphocyte count




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download