Abstract
Objectives
Outbreaks of pneumonia caused by Mycoplasma pneumoniae (MP) occur every 3-4 years in Korea, most recently in 2011. The aim of our study was to determine the optimal time to perform indirect particle agglutination antibody assays to improve early diagnosis of MP pneumonia in children.
Methods
A database of 206 pediatric patients treated for pneumonia at the Hanyang University Hospital from June to October 2011 was analyzed retrospectively for demographic characteristics and laboratory test results.
Results
Among the 206 patients treated for pneumonia during the study period, there were 160 children (mean age, 5.44 years) diagnosed with MP pneumonia, who were studied further. The mean age of these MP pneumonia patients was 5.44 years. Antibody titers increased with increasing time between symptom onset and the collection of serum collection: MP titers were <1:640 for sera collected after 5.44 days and titers ≥1:640 for those collected after 8.58 days; P<0.001). Antibody titers were considered positive when they reached ≥1:640. In 42 MP pneumonia patients in whom there was a four-fold or greater increase in titer between successive serum samples, the optimal cut-off time-point for distinguishing between the initial and second titer groups was 7.5 days after the onset of symptoms (sensitivity, 90.5%; specificity, 92.9%).
Figures and Tables
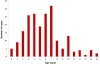 | Fig. 1Age distribution among children diagnosed with Mycoplasma pneumoniae pneumonia in the 2011 outbreak. |
 | Fig. 2Distribution of positive/negative Mycoplasma pneumoniae antibody titers according to time from symptom onset to serum collection. |
 | Fig. 4Receiver operating characteristics (ROC) curve to estimate the diagnostic sensitivity and specificity of Mycoplasma pneumoniae (MP) antibody testing of paired sera in children with MP pneumonia based on time from onset of symptoms. Arrow points to the optimum cut-off value: 7.5 days after symptom onset (sensitivity, 90.5%; specificity, 92.9%). |
 | Fig. 5Distribution of positive/negative Mycoplasma pneumoniae PCR results according to time from symptom onset to NP specimen collection. |
References
1. Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis. 1993; 17:Suppl. 1. S37–S46.
2. Bohte R, van Furth R, van den Broek PJ. Aetiology of community-acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax. 1995; 50:543–547.

3. Ou ZY, Zhou R, Wang FH, Lu JP, Xia JQ, Xia HM, et al. Retrospective analysis of Mycoplasma pneumoniae infection in pediatric fatal pneumonia in Guangzhou, South China. Clin Pediatr (Phila). 2008; 47:791–796.

4. Madani TA, Al-Ghamdi AA. Clinical features of culture-proven Mycoplasma pneumoniae infections at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. BMC Infect Dis. 2001; 1:6.
5. Lind K, Benzon MW, Jensen JS, Clyde WA. A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946-1995. Eur J Epidemiol. 1997; 13:581–586.
6. Foy HM, Kenny GE, Cooney MK, Allan ID. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979; 139:681–687.
7. Ito I, Ishida T, Osawa M, Arita M, Hashimoto T, Hongo T, et al. Culturally verified Mycoplasma pneumoniae pneumonia in Japan: a long-term observation from 1979-99. Epidemiol Infect. 2001; 127:365–367.

8. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008; 56:326–331.

9. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004; 17:697–728.

10. Martínez MA, Ruiz M, Zunino E, Luchsinger V, Avendano LF. Detection of Mycoplasma pneumoniae in adult community-acquired pneumonia by PCR and serology. J Med Microbiol. 2008; 57:1491–1495.

11. Zhang L, Zong ZY, Liu YB, Ye H, Lv XJ. PCR versus serology for diagnosing Mycoplasma pneumoniae infection: a systematic review & meta-analysis. Indian J Med Res. 2011; 134:270–280.
12. Waris ME, Toikka P, Saarinen T, Nikkari S, Meurman O, Vainionpaa R, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998; 36:3155–3159.

13. Barker CE, Sillis M, Wreghitt TG. Evaluation of Serodia Myco II particle agglutination test for detecting Mycoplasma pneumoniae antibody: comparison with mucapture ELISA and indirect immunofluorescence. J Clin Pathol. 1990; 43:163–165.

14. Jacobs E. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin Infect Dis. 1993; 17:suppl 1. S79–S82.
15. Loens K, Goossens H, Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010; 29:1055–1069.

16. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008; 32:956–973.

17. Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther. 2008; 6:509–521.
18. Youn YS, Lee KY. Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2012; 55:42–47.
19. Nagayama Y, Sakurai N, Yamamoto K, Honda A, Makuta M, Suzuki R. Isolation of Mycoplasma pneumoniae from children with lower-respiratory-tract infections. J Infect Dis. 1988; 157:911–917.

20. Kim NH, Lee JA, Eun BW, Shin SH, Chung EH, Park KW, et al. Comparison of polymerase chain reaction and the indirect particle agglutination antibody test for the diagnosis of Mycoplasma pneumoniae pneumonia in children during two outbreaks. Pediatr Infect Dis J. 2007; 26:897–903.

21. Parrott RH, Kim HW, Arrobio JO, Hodes DS, Murphy BR, Brandt CD, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973; 98:289–300.
22. Carlsen KH, Orstavik I, Halvorsen K. Viral infections of the respiratory tract in hospitalized children. A study from Oslo during a 90 months' period. Acta Paediatr Scand. 1983; 72:53–58.

23. Foy HM, Grayston JT, Kenny GE, Alexander ER, McMahan R. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA. 1966; 197:859–866.

24. Fernald GW, Collier AM, Clyde WA Jr. Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics. 1975; 55:327–335.
25. Alexander ER, Foy HM, Kenny GE, Kronmal RA, McMahan R, Clarke ER, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a cooperative medical group. N Engl J Med. 1966; 275:131–136.
26. Layani-Milon MP, Gras I, Valette M, Luciani J, Stagnara J, Aymard M, et al. Incidence of upper respiratory tract Mycoplasma pneumoniae infections among outpatients in Rhône-Alpes, France, during five successive winter periods. J Clin Microbiol. 1999; 37:1721–1726.

27. Principi N, Esposito S, Blasi F, Allegra L. Mowgli study group. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001; 32:1281–1289.

28. Kenny GE, Kaiser GG, Cooney MK, Foy HM. Diagnosis of Mycoplasma pneumoniae pneumonia: sensitivities and specificities of serology with lipid antigen and isolation of the organism on soy peptone medium for identification of infections. J Clin Microbiol. 1990; 28:2087–2093.

29. Thurman KA, Walter ND, Schwartz SB, Mitchell SL, Dillon MT, Baughman AL, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis. 2009; 48:1244–1249.

30. Hong JY, Na SY, Nam SG, Choi EH, Park JY, Lee HJ. Occurrence of Mycoplasma pneumoniae pneumonia in Seoul, Korea, from 1986 to 1995. J Korean Pediatr Soc. 1997; 40:607–613.
31. Thacker WL, Talkington DF. Analysis of complement fixation and commercial enzyme immunoassays for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin Diagn Lab Immunol. 2000; 7:778–780.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download