Abstract
Purpose
The aim for this study was to investigate clinical manifestation of seasonal influenza A and B during the 2012 winter season in Wonju, South Korea. Their clinical and laboratorial characteristics and effect of oseltamivir were compared and analyzed.
Methods
Children under the age of 18 years who visited the Wonju Severance Christian Hospital with fever or acute respiratory symptoms and who were diagnosed with influenza A or B by rapid antigen test from nasopharyngeal swab were selected for the study. The medical records of patients were retrospectively reviewed.
Results
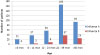
Influenza A was detected in 374 patients (83.7%), and influenza B in 72 (16.6%). The incidence of influenza A was highest in February (n=186), while that of influenza B was highest in March (n=36). The most common symptoms were fever (n=434, 97.1%) and cough (n=362, 81.0%). No significant differences were observed between influenza A and B in symptoms and laboratory data. Patients who had used oseltamivir within 2 days showed statistically lower admission rate, shorter admission duration, and lower incidence of pneumonia.
Figures and Tables
References
1. Korea Centers for Disease Control and Prevention. Korean influenza surveillance report, 2011-2012. Public Health Weekly Report;2012. 5:873–881. accessed on 12 Sep 2013. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU0004-MNU0036-MNU0037.
2. Wright P. Influenza viruses. In : Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 19th ed. Philadelphia: WB Saunders;2011. p. 1121–1125.
3. Ginocchio CC, Zhang F, Manji R, Arora S, Bornfreund M, Falk L, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009; 45:191–195.

4. Blyth CC, Iredell JR, Dwyer DE. Rapid-test sensitivity for novel swine-origin influenza A(H1N1) virus in humans. N Engl J Med. 2009; 361:2493.

5. Kim WJ. Epidemiology, clinical manifestations, and management of pandemic novel Influenza A (H1N1). Korean J Med. 2009; 77:157–164.
6. Wie SH, So BH, Song JY, Cheong HJ, Seo YB, Choi SH, et al. A comparison of the clinical and epidemiological characteristics of adult patients with laboratory-confirmed influenza A or B during the 2011-2012 influenza season in Korea: a multi-center study. PLoS One. 2013; 8:e62685.

7. Kim SH, Park CH, Huh K, Shim GH, Kim HB, You SJ, et al. Comparison of clinical manifestation and laboratory findings between H1N1 and influenza B infection. Pediatr Allergy Respir Dis. 2012; 22:64–70.

8. Yang SI, Rho JH, Sun YH, Cho KH, Shim SY, Eun BW, et al. The comparison of clinical characteristics and courses of pediatric patients hospitalized with pandemic influenza A (H1N1) and seasonal influenza from 2009 to 2011. Pediatr Allergy Respir Dis. 2012; 22:292–301.

9. Kawai N, Ikematsu H, Iwaki N, Kawashima T, Maeda T, Mitsuoka S, et al. Longer virus shedding in influenza B than in influenza A among outpatients treated with oseltamivir. J Infect. 2007; 55:267–272.

10. Sato M, Saito R, Sato I, Tanabe N, Shobugawa Y, Sasaki A, et al. Effectiveness of oseltamivir treatment among children with influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku J Exp Med. 2008; 214:113–120.

11. Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database Syst Rev. 2012; 4:CD002744.

12. Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res. 2013; 98:174–185.

13. de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005; 353:2667–2672.

14. Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the united states. JAMA. 2009; 301:1034–1041.

15. Seo ES, Park GH, Kim SM, Kim SW, Jung WS, Cho KS, et al. Oseltamivir efficacy, side effects, and safety in children with influenza. Korean J Pediatr. 2010; 53:56–66.

16. Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997; 87:1944–1950.

17. Thompson WW, Moore MR, Weintraub E, Cheng PY, Jin X, Bridges CB, et al. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009; 99:Suppl 2. S225–S230.

18. van den Wijngaard CC, van Asten L, Meijer A, van Pelt W, Nagelkerke NJ, Donker GA, et al. Detection of excess influenza severity: associating respiratory hospitalization and mortality data with reports of influenza-like illness by primary care physicians. Am J Public Health. 2010; 100:2248–2254.

19. Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, et al. Comparison of clinical features and outcomes of medically attended influenza A and influenza B In a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respir Viruses. 2012; 6:37–43.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download