Abstract
Purpose
Serotyping pneumococcal isolates is important to monitor efficacy of pneumococcal vaccines. Because of difficulties of typing pnueumocci, a multiplex bead-based (multibead) serotyping assay was recently introduced. The aim of this study is to establish a new multibead serotyping assay and to apply this method to analyze clinical isolates of pneumococci in Korea.
Methods
To establish the multibead serotyping assay, six key reagents were transferred from University of Alabama at Birmingham (UAB) to Ewha Center for Vaccine Evaluation and Study (ECVES): bead set coated with polysaccharide and monoclonal antibody pool were used in one multiplex inhibition—type immunoassay and 2 bead sets coated DNA probe and 2 primer pools were used in two multiplex PCR—based assays. After multibead serotyping assay was set up, 75 test samples of pneumococci were analyzed whether ECVES is able to identify serotype correctly. After confirming the performance, serotyping assay was applied to identify serotypes of 528 clinical isolates of pneumococci collected from 3 different hospitals.
Results
After establishment of the multibead pneumococcal serotyping assay system at ECVES, 75 test samples were analyzed. There was no discrepancy of serotypes of 75 test samples between the results assigned at UAB and those at ECVES. The serotypes of 528 pneumococci isolated from patients or healthy subjects were determined in 94.3% of isolates (498/528).
Go to : 
REFERENCES
1. Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbi—nowitsch E, Collins M, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006; 2:e31.

2. CaliX JJ, Nahm MH. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis. 2010; 202:29–38.
3. Park 1H. Geno KA, Yu J, Oliver MB, Kim KH, Nahm MH. Genetic, biochemical, and serological characterization of a new pneumococcal serotype, 6H, and generation of a pneu—mococcal strain producing three different capsular repeat units. Clin Vaccine Immunol. 2015; 22:313–8.
4. Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non—pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vacci—nation, 1998—2004. J Infect Dis. 2007; 196:1346–54.

5. Lund E. Laboratory diagnosis of pneumococcus infections. Bull World Health Organ. 1960; 23:5–13.
6. Slotved HC, Kaltoft M, Skovsted IC, Kerrn MB, Espersen F. Simple, rapid latex agglutination test for serotyping of pneu—mococci (Pneumotest—Latex). J Clin Microbiol. 2004; 42:2518–22.

7. Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneu— monjae isolates. J Clin Microbiol. 2006; 44:124–31.
8. Magomani V, Wolter N, Tempia S, du Plessis M, de Gouveia L, von Gottberg A. Challenges of using molecular serotyping for surveillance of pneumococcal disease. J Clin Microbiol. 2014; 52:3271–6.

9. Yu J, Lin J, Benjamin WH Jr, Waites KB, Lee CH, Nahm MH. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J Clin Microbiol. 2005; 43:156–62.

10. Yu J, Lin J, Kim KH, Benjamin WH Jr, Nahm MH. Develop—ment of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin Vaccine Immunol. 2011; 18:1900–7.

11. Yu j. Carvalho Mda G, Beall B, Nahm MH. A rapid pneu—mococcal serotyping system based on monoclonal antibodies and PCR. J Med Micr0b101. 2008; 57:171–8.
12. Turner P, Turner C, Iankhot A, Phakaudom K, Nosten F, Goldblatt D. Field evaluation of culture plus latex sweep serotyping for detection of multiple pneumococcal serotype colonisation in infants and young children. PLoS One. 2013. 81667933.

13. Siira L, Kaijalainen T, Lambertsen L, Nahm MH, Toropainen M, Virolainen A. From Quellung t0 multiplex PCR, and back when needed, in pneumococcal serotyping. j Clin Microbiol. 2012; 50:2727–31.
14. Dhoubhadel BG, Yasunami M, Yoshida LM, Thi HAN, Thi THV, Thi TAN, et al. A novel high—throughput method for molecular serotyping and serotype—specific quantification of Streptococcus pneumoniae using a nanofluidic real—time PCR system. J Med Microbiol. 2014; 63:528–39.
15. Findlow H, Laher G, Balmer P, Broughton C, Carrol ED, Borrow R. Competitive inhibition flow analysis assay for the non—culture—based detection and serotyping of pneumococcal capsular polysaccharide. Clin Vaccine Immunol. 2009; 16:222–9.
16. Cho EY, Lee H, Choi EH, Kim Yj, Eun BW, Cho YK, et al. Serotype distribution and antibiotic resistance of Streptococ— cus pneumoniae isolated from invasive infections after optional use of the 7-Valent conjugate vaccine in Korea, 2006—2010. Diagn Microbiol Infect Dis. 2014; 78:481–6.
17. Hathaway LJ, Stutzmann Meier P, Battig P, Aebi S, Muhle-mann K. A homologue of aliB is found in the capsule region of nonencapsulated Streptococcus pneumoniae. J Bacteriol. 2004; 186:3721–9.
18. Nagai K, Shibasaki Y, Hasegawa K, Davies TA, Jacobs MR, Ubukata K, et al. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and βlactam resistance, and to detect common macrolide resistance determinants. J Antimicrob Chemother. 2001; 48:915–8.
19. Kawaguchiya M, Urushibara N, Ghosh S, Kuwahara O, Morimoto S, Ito M, et al. Serotype distribution and suscepti—bility to penicillin and erythromycin among noninvasive or colonization isolates of Streptococcus pneumoniae in northern Japan: A cross—sectional study in the pre-PCV7 routine immunization period. Microb Drug Resist. 2014; 20:456–65.
20. Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007; 45:1225–33.
Go to : 
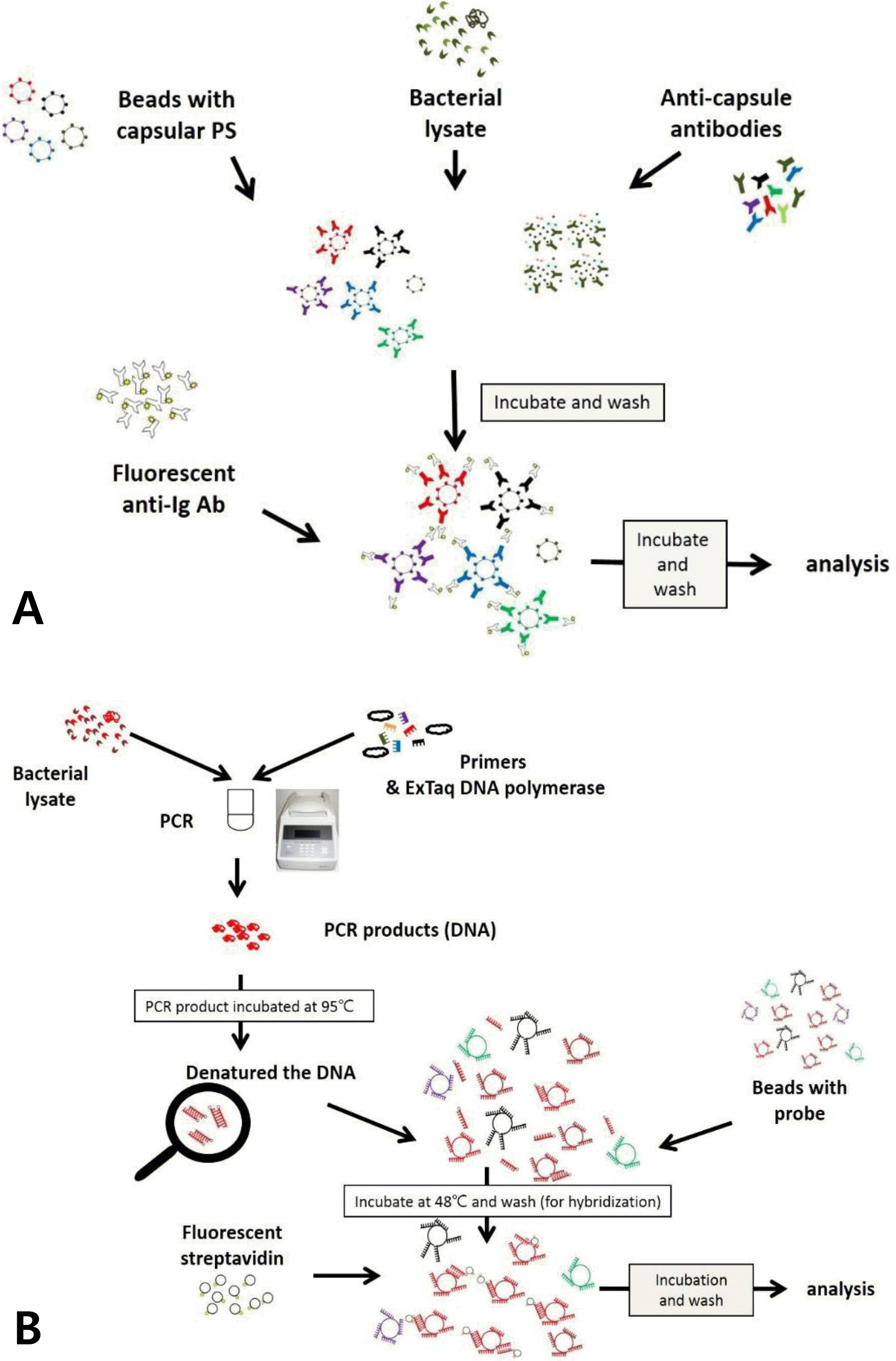 | Fig. 1.Scheme of multibead serotyping assay procedure. (A) Multibead serotyping assay using monocional antibodies (Reaction A). In this assay, the bacterial lysates and a mixture of color—coded beads coupled to reference capsular polysaccharides (PSs) are incubated with a mixture of monocional antibodies that bind the immobilized capsular PSs. If a capsular P5 is in the sample, the PS will inhibit the binding of the monoclonal antibody to the corresponding PS—coated bead. The amount of the monoclonal antibody bound to the beads is determined using a fluorescently labeled anti—mouse immunoglobulin secondary antibody. (B) Multibead serotyping assay using wzy PCR (Reaction B and C). The assay is designed to detect pneumococcal serotypes by identifying wzy gene. wzy from a pneumococcal lysate is PCR amplified with a mixture of PCR primers and the resulting PCR product is identified by hybridizing it to luminex beads which are conjugated with a serotype specific probe. |
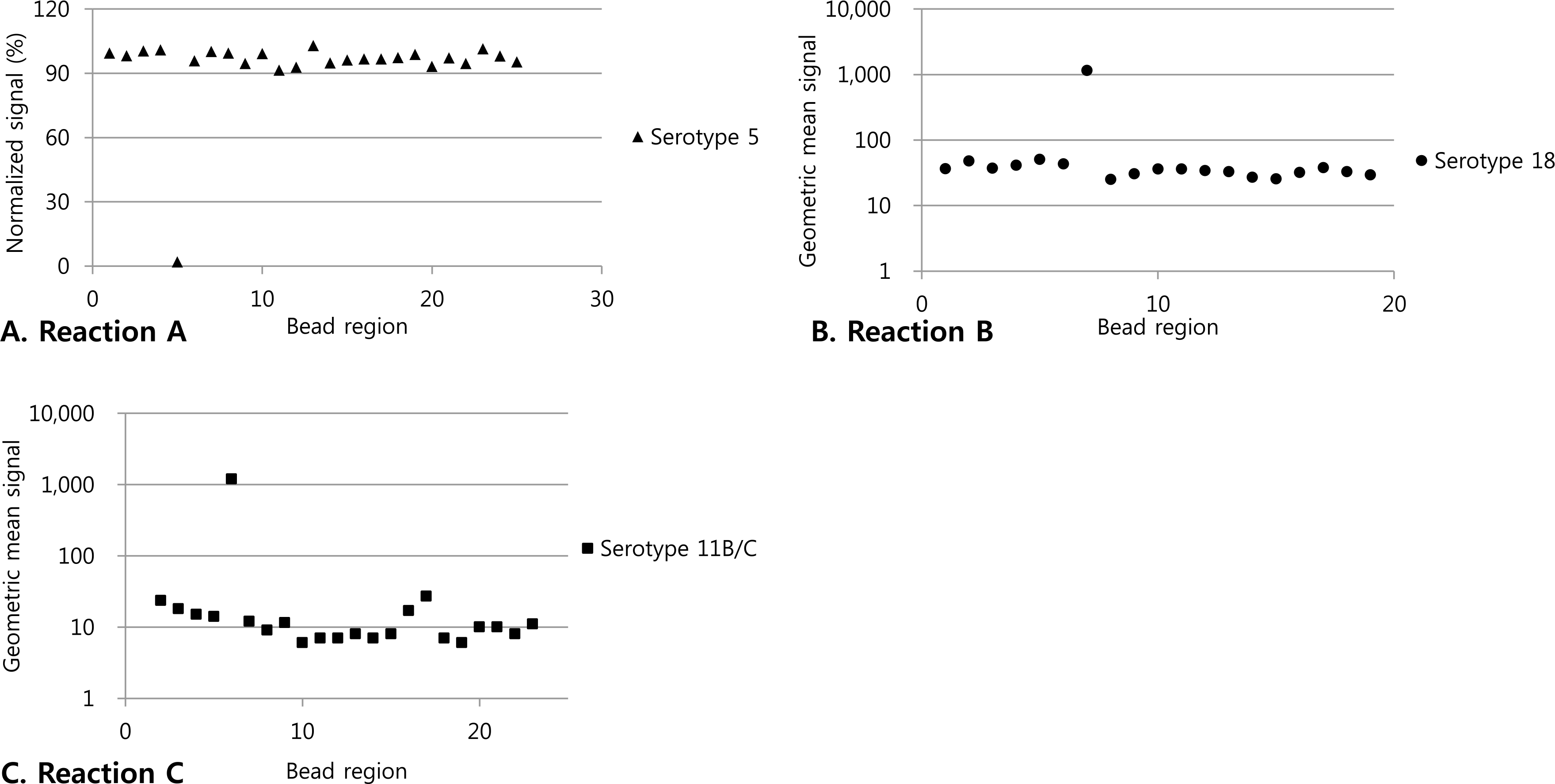 | Fig. 2.Specificity of multibead serotyping assay. (A) Normalized fluorescence signals of the beads coated with pneumococcal polysaccharides obtained with the panel of a test sample by use of the multiplexed inhibition type immunoassay with monoclonal antibodies (Reaction A). The numbers on the X axis represents the numbers assigned to the bead regions in the test. (B and C) Geometric mean signal of the beads coated with probes obtained with the panel of each sample by use of the PCR-based multiplexed assay (Reaction B and C). The numbers on the X axis represents the numbers assigned to the bead regions in the test. |
Table 1.
Bead Regions and Serotype Specificity in Reaction A, B and C
Table 2.
Serotyping Results Deduced bv Multibead Serotyping Assav 10f 528 Clinical Isolates




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download