Abstract
Purpose
The study aimed to determine data collected during tuberculosis (TB) contact investigations and to evaluate the outcomes of these investigations.
Methods
We reviewed medical records for child contacts of patients with culture-positive pulmonary TB aged 19 years or older between August 2012 and July 2014.
Results
A total of 116 child contacts were identified for 79 patients with culture—positive pulmonary TB. of 116 contacts identified, 22% were incompletely screened. Of 90 contacts who completed screening, 42% had negative tuberculin skin test (TST) results, 58% had positive results, and 1% had active pulmonary TB at the time of investigation. Of 50 contacts with TB patients with a negative smear, 50% had positive TST results. Age 25 years (OR 8.3; 95% CI 2.3-30) and male gender (OR 3.9; 95% CI 1.5—9.9) were significantly associated with being incompletely screened.
REFERENCES
1. Korea Centers for Disease Control and Prevention. Elimina—tion of TB 2030. Reston: Korea Centers for Disease Control and Prevention;2008.
2. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on child—hood tuberculous meningitis and miliary tuberculosis world—wide: a meta—analysis and assessment of cost-effectiveness. Lancet. 2006; 367:1173–80.

3. Centers for Disease Control and Prevention. Targeted tuber—culin testing and treatment of latent tuberculosis infection. MMWR. 2000; 49(RR—6):1–51.
4. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974; 99:131–8.

5. Joint Committee for the Development of Korean Guidelines for Tuberculosis. Korean guidelines for tuberculosis, Ist ed. Reston: Korea Centers for Disease Control and Prevention;2011.
6. Korea Centers for Disease Control and Prevention. National Guidelines for Control and Prevention of Tuberculosis. Reston: Korea Centers for Disease Control and Prevention;2015.
7. Centers for Disease Control and Prevention. Essential com—ponents of a tuberculosis prevention and control program: recommendations of the Advisory Council for the Elimina—tion of Tuberculosis. MMWR. 1995; 44(R—11):2.
8. Marks SM, Taylor Z, Qualls NL, Shrestha—Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000; 162:2033–8.

9. Reichler MR, Reves R, Bur S, Thompson V, Mangura BT, Ford J, et al. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002; 287:991–5.

10. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investiga—tion for tuberculosis: a systematic review and meta—analysis. Eur Respir J. 2013; 41:140–56.

11. Veen J. Microepidemics of tuberculosis: the stone—in—the—pond principle. Tuber Lung Dis. 1992; 73:73–6.

13. National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tubercu—losis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR. 2005; 54(RR—15):1–47.
14. Joint Tuberculosis Committee of the British Thoracic Society. Control and prevention of tuberculosis in the United King—dom: code of practice 2000. Thorax. 2000; 55:887–901.
Fig. 1.
Flowchart of child contact investigations of infectious tuberculosis patients, Seongnam, Korea, 2012-2014. ∗There were 11 LTBI patients who were not offered treatment because of contact to multidrug—resistantTB patients or parental refusal of treatment. TST indi—cates tuberculin skin test.
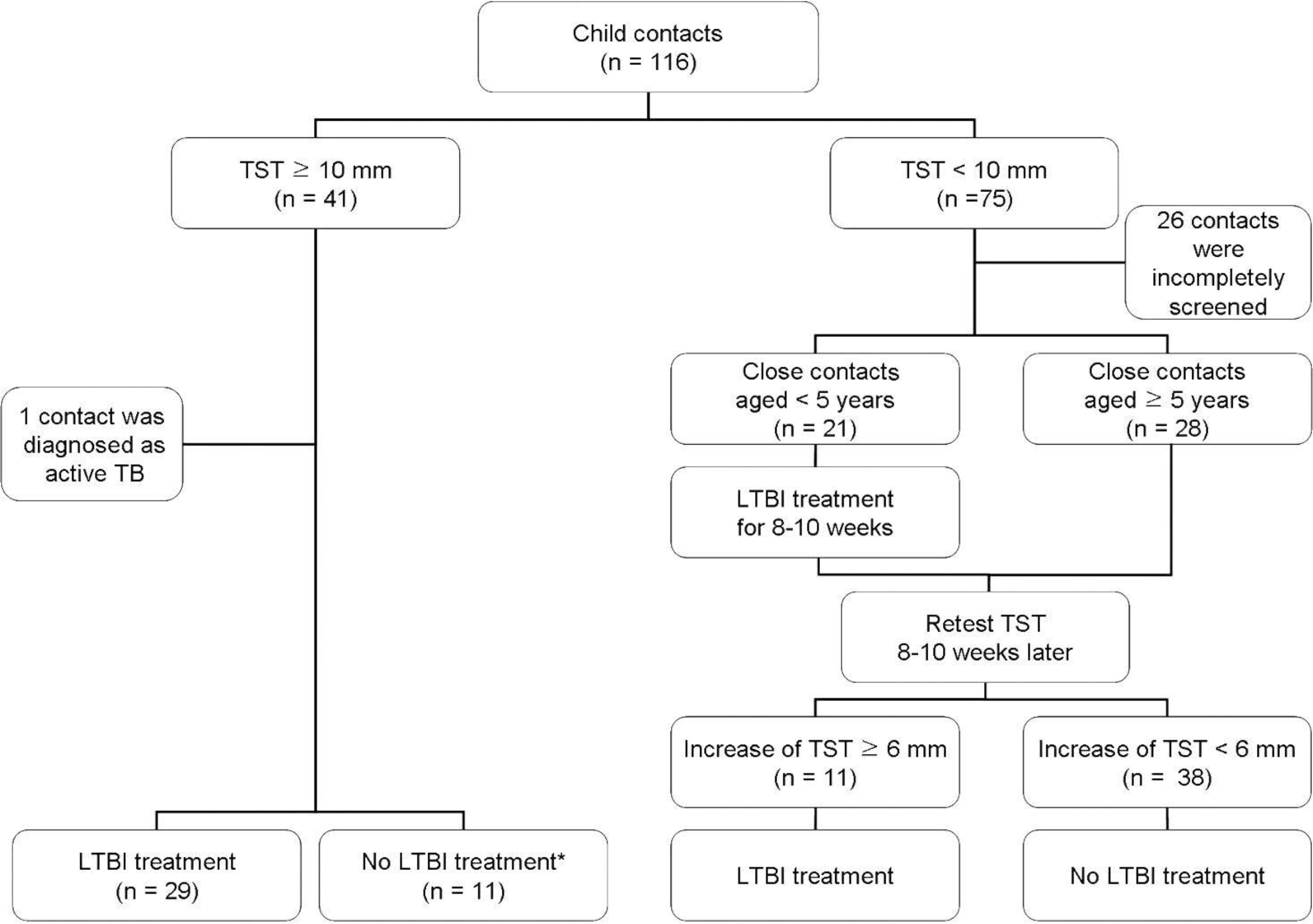
Fig. 2.
Proportion of child contacts with positive tuberculin skin test (TST) results by bacteriologic status of index cases. AFB indicated acid-fast bacilli.
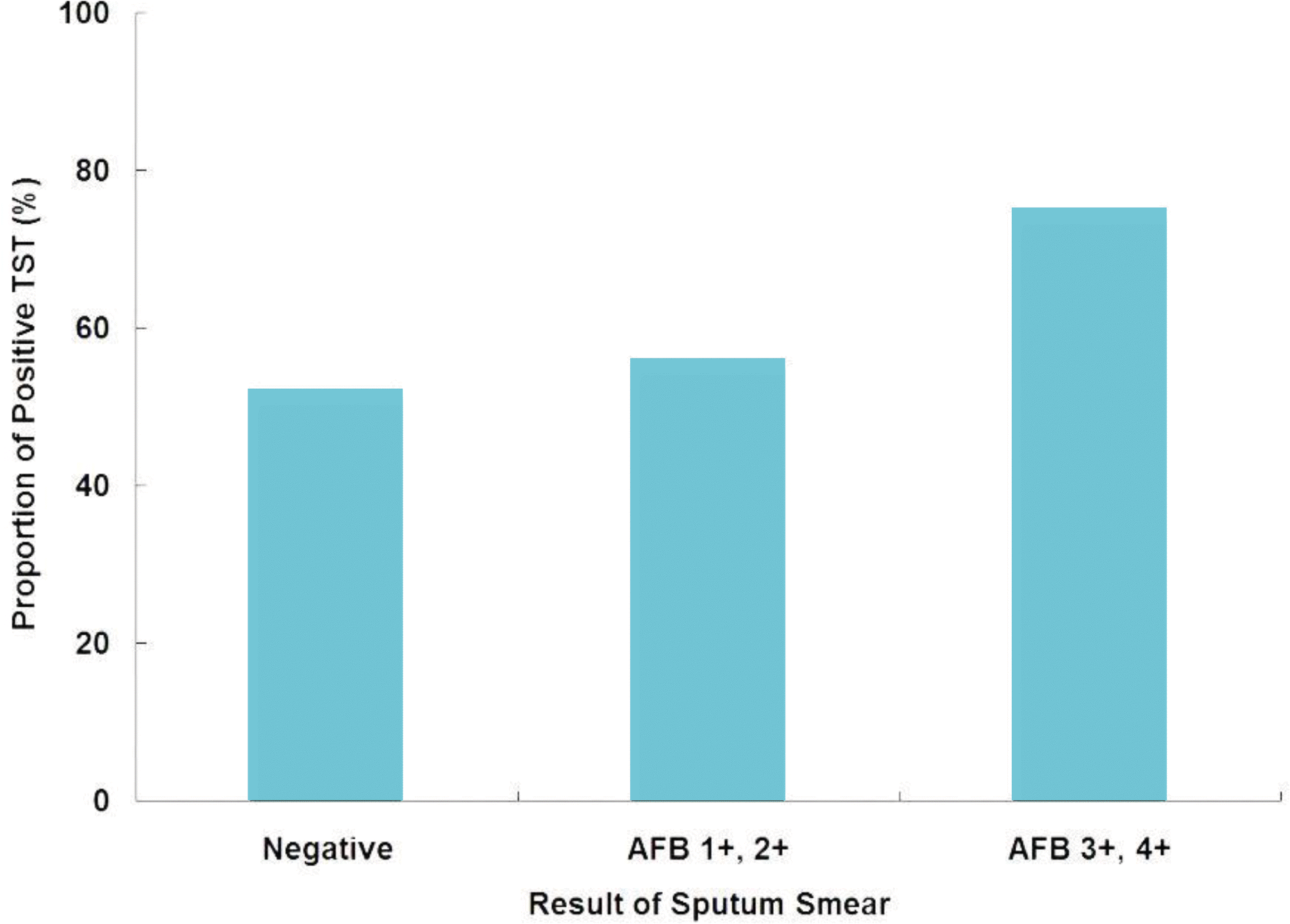
Table 1.
Multivariate Regression Model for Predictors of child contracts Who Were Incompletely Screened




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download