Abstract
Purpose
In this study, doctors were surveyed with a questionnaire to determine whether they performed simultaneous vaccination and whether there were any concerns about safety or anxiety. The purpose of this study was to determine any problems associated with doctors readily performing simultaneous vaccination.
Methods
A trained surveyor visited 241 doctors from every institution registered with the National Immunization Program (NIP) located within six districts (gu) in the City of Busan (Dongnae—gu, Geumjeong—gu, Yeonje—gu, Suyeong—gu, Busanjin—gu, Haeundae—gu); a total of 155 (64%) valid responses were obtained.
Results
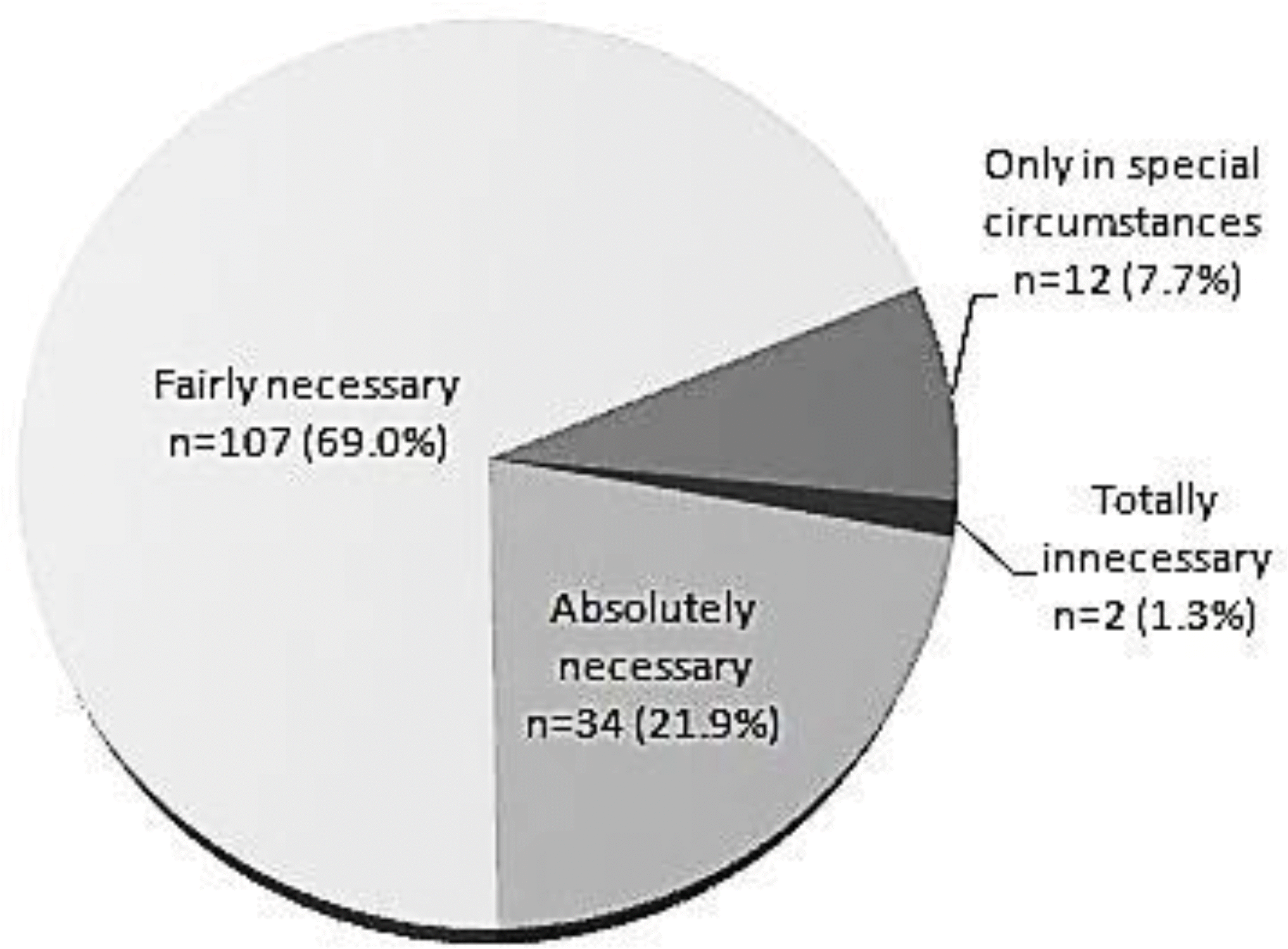
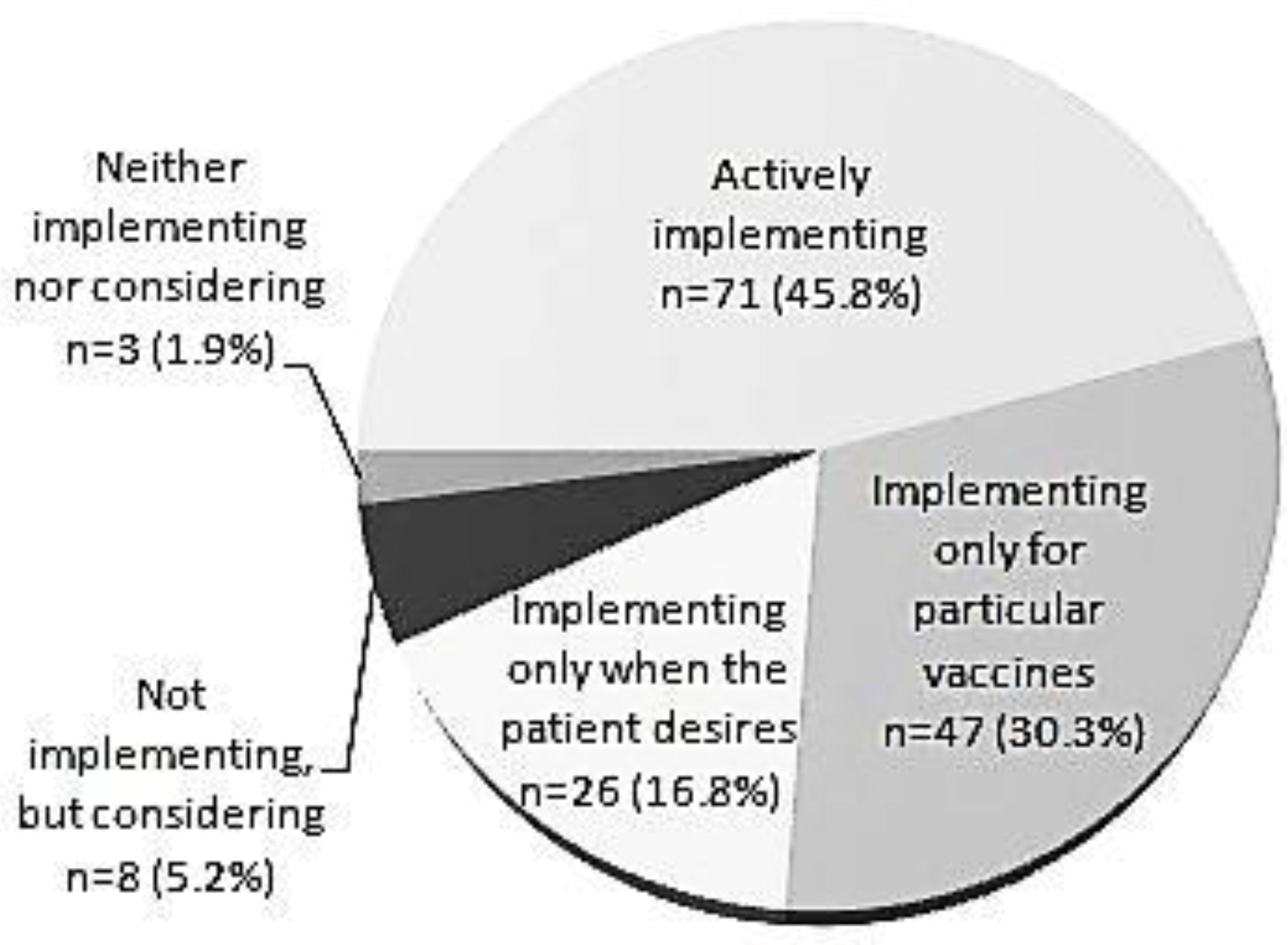
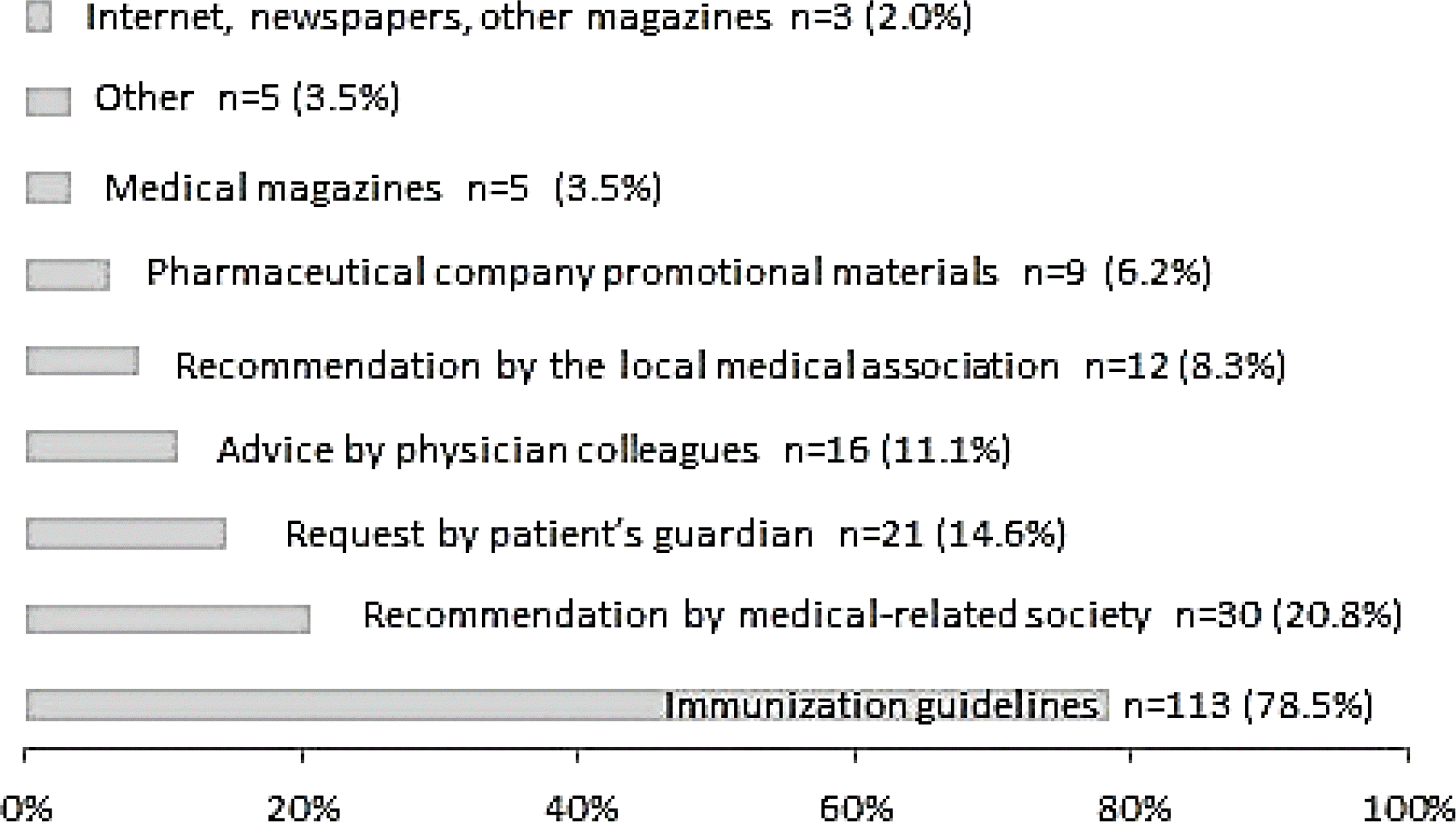
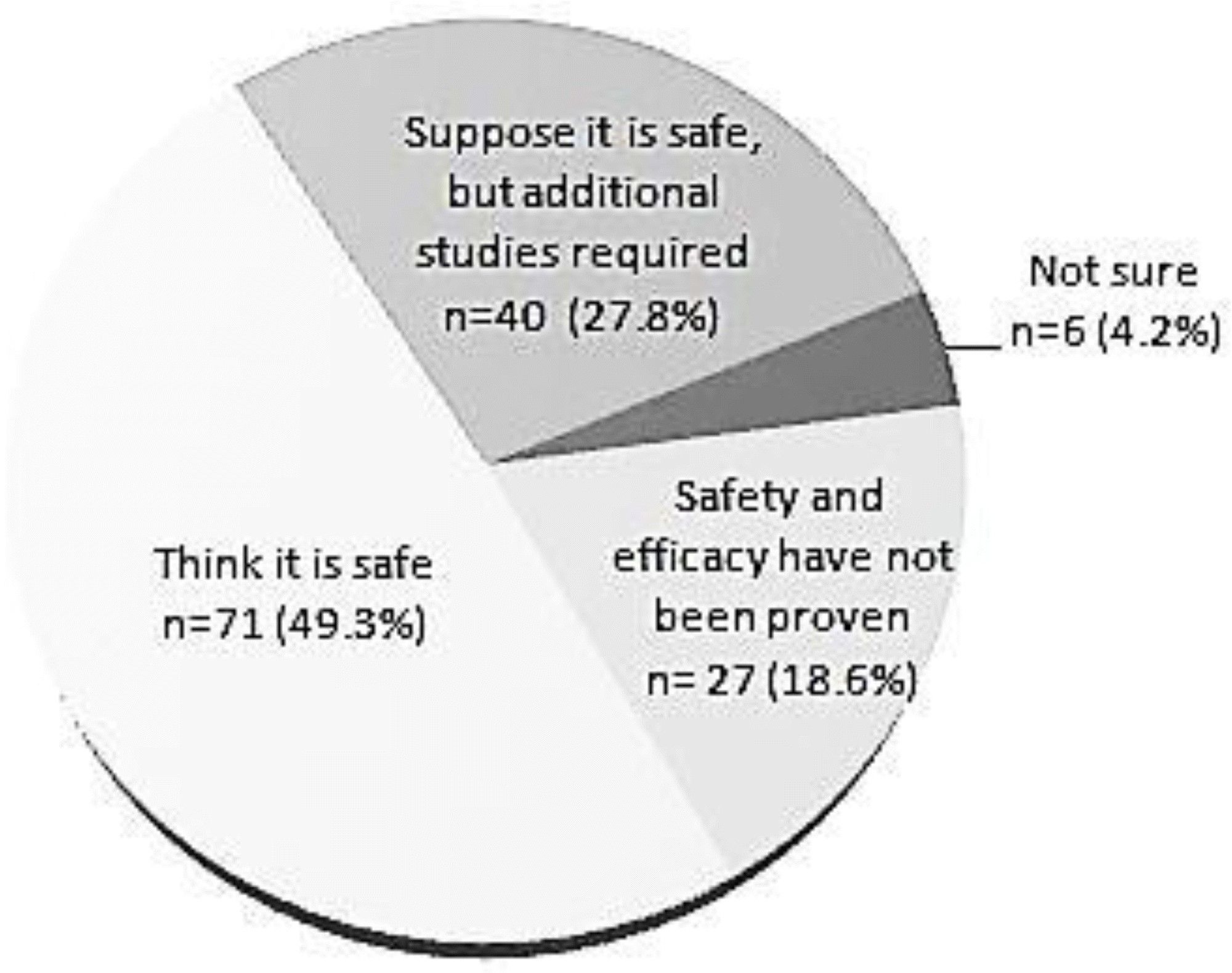
Of the 155 respondents, 144 (93%) were already performing simultaneous immunizations and 141 (91%) had a positive view of the practice. However, among the 144 doctors performing simultaneous immunizations, 67 (47%) were not confident about its safety; side effects were seen after simultaneous immunization by 86 doctors, 35 (41%) of whom believed that the frequency or possibility of side effects in simultaneous immunizations was higher than that in sequential immunizations.
Conclusions
The use of simultaneous immunization is expanding quickly. However, among the doctors performing simultaneous immunizations, a high percentage had concerns over its unproven safety and potential side effects, indicating the need for academic societies or government institutions to present evidence to address such concerns.
Go to : 
REFERENCES
1. long KL, Choi WS. Immunization policy in Korea. Infect Chemother. 2008; 40:14–23.
2. Advisory Committee on Immunization Practices (ACIP). General recommendations on immunization. Recommen—dations and Reports. 2011; 60:1–64.
3. Dubai health authority. Immunization guidelines. Dubai: Department of Public Health & Safety Policy & Strategy Sector. 2012. 1–79.
4. Public Health Agency of Canada. Canadian immunization guide. Canadian Immunization Guide part 1. 2014. 1–131.
5. The Korean Pediatric Society. Immunization guideline. Immunization guideline 7th edition. 2012. 21–309.
6. Hanna IN, Bullen RC, Andrews DE. The acceptance of three simultaneous vaccine injections recommended at 12 months of age. Commun Dis Intell Q Rep. 2004. 493–6.
7. Katsuta T, Miyazi Y, Nakamura Y, Tsuruoka Z, Tateyama S, Tokutake T, et al. A survey of doctors’ awareness of simultaneous vaccination in Japan. Japan J Pediatr. 2013; 117:1645–51.
8. Rhim 1W. Kim CH, Lee WB, Kang IH. A survey of parental knowledge ofvaccination. Korean I Pediatr. 2006; 49:251–7.
9. Paik SH, Chung EH, Uhm MR, Shin SM, Lee WG, Lee MN, et al. A questionnaire on using informed consents of parents or guardians in vaccination of children. Korean I Pediatr. 2003; 46:647–54.
10. Hong Y]. Combination vaccines. Korean J Pediatr. 2003; 46:213–6.
11. Offit PA, Quarles I, Gerber MA, Hackett C], Marcuse EK, Kollman TR, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant's immune system? Pediatrics J. 2002. 109–24.

12. Meyerhoff AS, Weniger MG, Iacobs RJ. Economic value to parents of reducing the pain and emotional distress of child—hood vaccine injections. Pediatr Infect Dis I. 2001; 20:57–62.

13. Oh SH. Active immunization. Hanyang Medical Reviews. 2008; 28:4–15.
14. Ju HG, Choi YY, Ma JS, Hwang TJ. Effect of simultaneous administration of hepatitis B vaccine with DPT and oral polio vaccine. Korean J Pediatr. 1998; 41:451–5.
15. Melman ST, Nguyen TT, Ehrlich E, Schorr M, Anbar RD. Parental compliance with multiple immunization injections. Arch Pediatr Adolesc Med. 1999; 153:1289–91.

16. Kim CH. Combination vaccines. Korean J Pediatr. 2000; 43:322–5.
17. McGowan A, Cottrell S, Roberts R, Lankshear A. Minimising pain response during routine infant imminisation. Commu—nity Pract. 2013; 86:24–8.
18. Olin P, Rasmussen F, Gottfarb P. Schedules and protection, simultaneous vaccination and safety: Experiences from recent controlled trials. IntJ Infect Dis. 1997. 12143–7.

19. Shneyer E, Strulov A, Rosenfeld Y. Reduced rate of side effects associated with separate administration on MMR and DTaP—Hib—IPV vaccinations. Isr Med Assoc J. 2009; 11:735–8.
20. Jo DS, Choi EH. General recommendations for immunization practices in children and adolescents. J Korean Med Assoc. 2009; 52:225–32.

21. Engineer FG, Keskinocak P, Pickering LK. Catch-up schedu—ling for childhood vaccination. Operations Research. 2009; 57:1307— 19.
22. Anderson RS, Healey ML. Immunization rates in children receiving Diphtheria—TetanusPertussis and Measles—Mumps—Rubella vaccines simultaneously. J Child Fam Nurs. 2000; 7:27–38.
23. Advisory Committee on Immunization Practices (ACIP). Adolescent immunizations: A position paper of the society for adolescent medicine. J Adolesc Health. 2006. 321–7.
Go to : 
Table 1.
Demographic Characteristics of the Survey Respondents




 PDF
PDF ePub
ePub Citation
Citation Print
Print


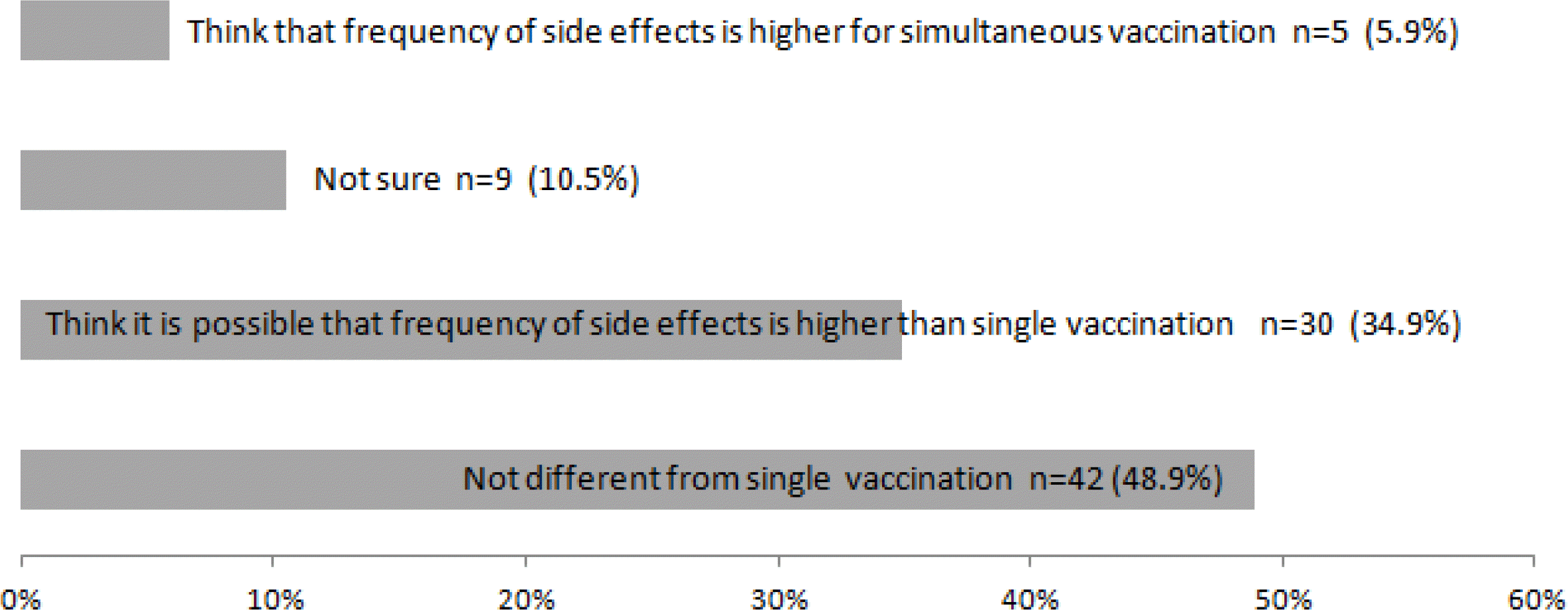
 XML Download
XML Download