Abstract
Purpose
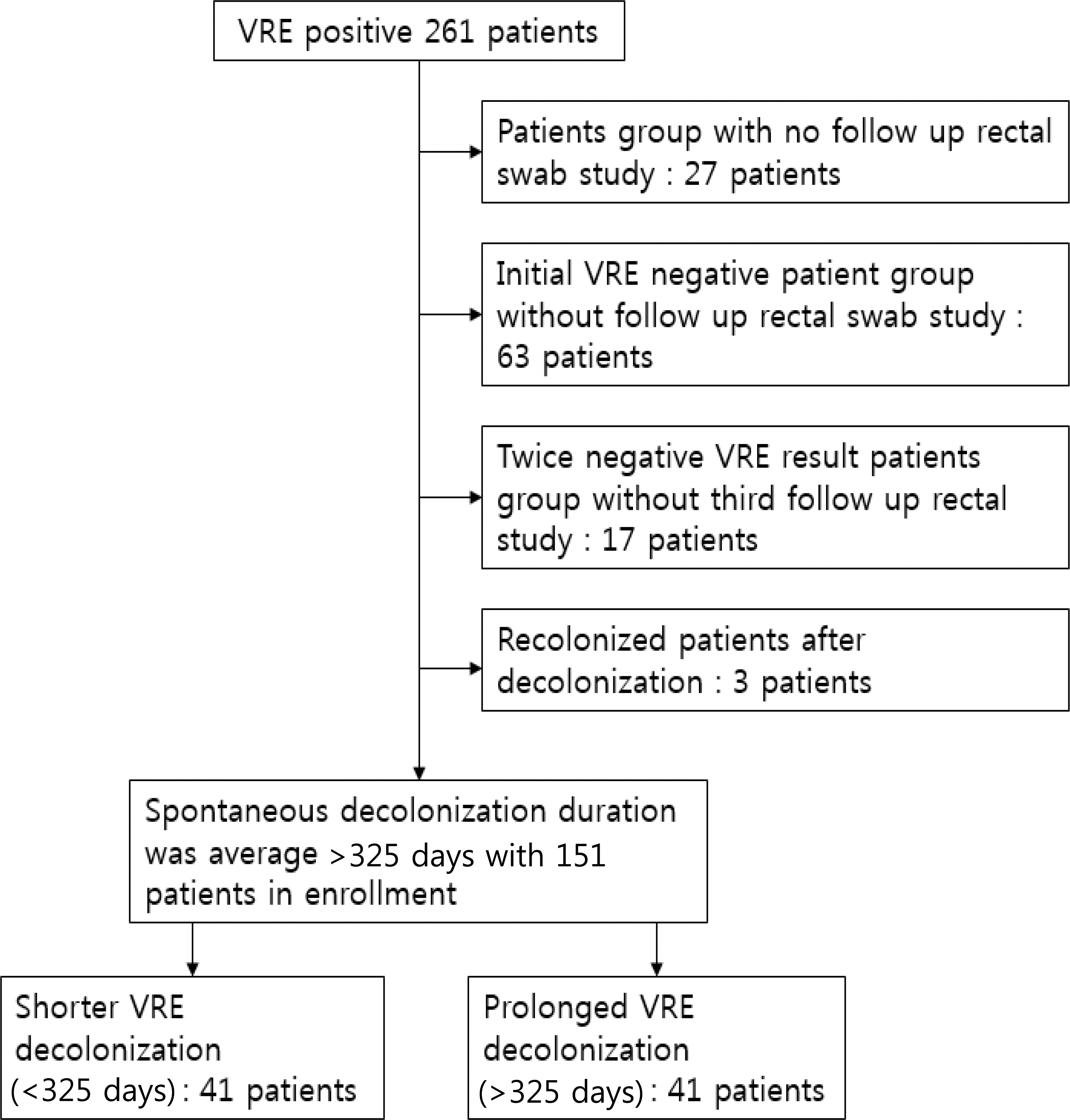
The aim of this study was to identify the factors influencing the spontaneous decolonization period of vancomycin resistant enterococcus (VRE) species in pediatric patients. Methods: The medical records of patients presenting positive VRE cultures between January 2005 and November 2010 at a tertiary hospital in Seoul, Korea, were reviewed retrospectively. The subjects were divided into two groups according to the average number of days for decolonization (325 days). Clinical characteristics were compared between shorter VRE colonization patients (<325 days, n=41) and prolonged VRE colonization patients (>325 days, n=110). Results: There were 151 patients who had more than 1 year of follow up period or confirmed of VRE decolonization among patients who were identified with VRE. The average age at the time of initial VRE colonization was significantly younger in shorter decolonization group than in prolonged decolonization group (44.9 months vs 40.9 months, P:0.040). The prolonged decolonization group received more vancomycin treatments after VRE colonization in comparison with patients in shorter decolonization group (7.0% vs 27.2%, P:0.008). Conclusions: For the duration of VRE colonization, it was found that the initial age of acquiring VRE and use of antibiotics were important factors. Antibiotics should be used properly and precisely in order to treat infectious diseases and to control the colonization of antibiotic resistant bacteria.
Go to : 
REFERENCES
1. Uttley AH, Collins CH, Naidoo I, George RC. Vancomycin—resistant enterococci. Lancet. 1988; 1:57–8.

2. Tenover FC, McDonald LC. Vancomycin—resistant staphylococci and enterococci: epidemiology and control. Curr Opin Infect Dis. 2005; 18:300–5.

3. Burrell LI, Grabsch EA, Padiglione AA, Grayson ML. Prevalence of colonisation with vancomycin-resistant enterococci (VRE) among haemodialysis outpatients in Victoria: implications for screening. Med I Aust. 2005. 1821492.

4. Martone WI. Spread of vancomycin—resistant enterococci: why did it happen in the United States? Infect Control Hosp Epidemiol. 1998; 19:539–45.

5. Streit IM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001). Int I Antimicrob Agents. 2004; 24:111–8.

6. Zirakzadeh A, Patel R. Vancomycin—resistant enterococci: colonization, infection, detection, and treatment. Mayo Clinic proceedings. Mayo Clinic. 2006; 81:529–36.
7. Sujatha S, Praharaj I. Glycopeptide resistance in gram—positive cocci: a review. Enferm Infecc Microbiol Clin. 2012; 2012:781679.

8. Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002; 162:2223–8.

9. Pelz RK, Lipsett PA, Swoboda SM, Diener—West M, Powe NR, Brower RG, et al. Vancomycin—sensitive and vancomycin—resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med. 2002; 28:692–7.

10. Song X, Srinivasan A, Plaut D, Perl TM. Effect of nosocomial vancomycin—resistant enterococcal bacteremia on mortality, length of stay, and costs. Infect Control Hosp Epidemiol. 2003; 24:251–6.

11. Chang S, Sievert DM, Hageman IC, Boulton ML, Tenover FC, Downes FP, et al. Infection with vancomycin—resistant Staphylococcus aureus containing the vanA resistance gene. N Engl I Med. 2003; 348:1342–7.
12. Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009; 53:4580–7.
13. Mazuski JE. Vancomycin—resistant enterococcus: risk factors, surveillance, infections, and treatment. Surg Infect (Larchmt). 2008; 9:567–71.
14. Kauffman CA. Therapeutic and preventative options for the management of vancomycin-resistant enterococcal infections. I Antimicrob Chemother. 2003; 51(Suppl 3):iii23–30.

15. Arias CA, Contreras GA, Murray BE. Management of mul-tidrug—resistant enterococcal infections. Ch'n Microbiol Infect. 2010; 16:555–62.

16. Linden PK. Treatment options for vancomycin—resistant enterococcal infections. Drugs. 2002; 62:425–41.
17. Montecalvo MA. Ramoplanin: a novel antimicrobial agent with the potential to prevent vancomycin-resistant enterococcal infection in high-risk patients. J Antimicrob Chemother. 2003; 51(Suppl 3):11131–5.

18. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Infect Control Hosp Epidemiol. 1995; 23:87–94.
19. Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009; 155:1749–57.

20. Yoon YK, Lee SE, Lee J, Kim H], Kim IY, Park DW, et al. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case—control study. I Antimicrob Chemother. 2011; 66:1831–8.

21. Baden LR, Thiemke W, Skolnik A, Chambers R, Strymish 1, Gold HS, et al. Prolonged colonization with vancomycin-re-sistant Enterococcus faecium in long—term care patients and the significance of "clearance". Clin Infect Dis. 2001; 33:1654–60.
22. Henning KI, Delencastre H, Eagan I, Boone N, Brown A, Chung M, et al. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis I. 1996; 15:848–54.

23. Byers KE, Anglim AM, Anneski CI, Farr BM. Duration of colonization with vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2002; 23:207–1. 1.
24. Manley KI, Fraenkel MB, Mayall BC, Power DA. Probiotic treatment of vancomycin—resistant enterococci: a randomised controlled trial. Med I Aust. 2007; 186:454–7.

25. Szachta P, Ignys I, Cichy W. An evaluation of the ability of the probiotic strain Lactobacillus rhamnosus GG to eliminate the gastrointestinal carrier state of vancomycin—resistant enterococci in colonized children. J Clin Gastroenterol. 2011; 45:872–7.

26. Sekirov 1. Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010; 90:859–904.
27. Donskey CI, Hoyen CK, Das SM, Helfand MS, Hecker MT. Recurrence of vancomycin—resistant Enterococcus stool colonization during antibiotic therapy. Infect Control Hosp Epidemiol. 2002; 23:436–40.
28. Donskey C]. Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan IA, Hujer AM, et al. Effect of antibiotic therapy on the density of vancomycin—resistant enterococci in the stool of colonized patients. N Engl I Med. 2000; 343:1925–32.
29. Harbarth S, Cosgrove S, Carmeli Y. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 2002; 46:1619–28.

30. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 1995; 44:1–13.
31. Muto CA, Iernigan IA, Ostrowsky BE, Richet HM, Iarvis WR, Boyce IM, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003; 24:362–86.
Go to : 
Table 1.
Comparison of Baseline Demographic and Clinical Characteristics with Shorter Vancomycin Resistant Enterococcus Colonization Group and Prolonged Vancomycin Resistant Enterococcus Colonization Group
Table 2.
Comparison of Antibiotic Use before Vancomycin Resistant Enterococcus Colonization between Prolonged Vancomycin Resistant Enterococcus Colonization Group and Shorter Vancomycin Resistant Enterococcus Colonization Group
Table 3.
Comparison of Antibiotic Use after Vancomycin Resistant Enterococcus Colonization between Prolonged Vancomycin Resistant Enterococcus Colonization Group and Shorter Vancomycin Resistant Enterococcus Colonization Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download