Abstract
Purpose
This study was aimed to investigate the contamination rates of blood culture in a neonatal intensive care unit (NICU) and to exa—mine the clinical characteristics related to the contamination. Methods: Eight hundred thirty cases of blood culture performed from March 2013 to February 2014 were analyzed. We evaluated the contamination rates of blood culture by blood sampling sites and compared the clinical characteristics such as real name system and body weights of the contaminated cases and those of non—contaminated ones. The clinical characteristics were retrospectively reviewed by medical records. Results: The overall contamination rate was 3.6% (30/830). The contamination rates by blood sampling sites were as follows: peripheral vein 15.6% (10/64), peripheral artery 2.6% (20/759), and umbilical arterial catheter 0% (0/7). There was no difference in the contamination rates between cases with and without real name system (P=O.484). However, there were significant differences in the contamination rates by the physicians who performed the culture (P=0.038) and body weight (<1,000 9 vs. 21,000 g) at the time of blood culture (P<0.001). Conclusions: These results suggest that neonates with a body weight less than 1,000 9 have more risks of the contamination of blood cul—ture. Furthermore, there is a necessity to provide blood culture performers with active feedbacks and individualized education plans that can help diminish blood culture contamination rates. Prospective studies in a systematic manner that can be applied in actual clinical settings are needed in order to figure out factors that can diminish the contamination rates of blood culture in NICU.
Go to : 
REFERENCES
1. Baron E]. Weinstein MP, Dunne WM Ir, Yagupsky P, Welch DF, Wilson DM. Cumitech 1C: Blood Cultures IV. Washington DC: ASM Press;2005.
2. Clinical and Laboratory Standards Institute. Principles and procedures for blood culture. Approved guideline. Document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute;2007.
3. Snyder SR, Favoretto AM, Baetz RA, Derzon jH, Madison BM, Mass D, et al. Effectiveness of practices to reduce blood culture contamination: a Laboratory Medicine Best Practices systematic review and meta-analysis. Clin Biochem. 2012; 45:999–101.

4. Lin CM, Lee WS, Lin FY, Yu FL, Ou TY, Teng SO. Reducing blood culture contamination rates by educational intervention and one-on-one feedback in the emergency department. I Exp Clin Med. 2012; 4:154–6.

5. Self WH, Speroff T, McNaughton CD, Wright PW, Miller G, johnson JG, et al. Blood culture collection through peripheral intravenous catheters increases the risk of specimen contamination among adult emergency department patients. Infect Control Hosp Epidemiol. 2012; 33:524–6.

6. Stohl S, Benenson S, Sviri S, Avidan A, Block C, Sprung CL, et al. Blood cultures at central line insertion in the intensive care unit: comparison with peripheral venipuncture. I Clin Microbiol. 2011; 49:2398–403.

7. Kim NH, Kim M, Lee S, Yun NR, Kim KH, Park SW, et al. Effect of routine sterile gloving 0n contamination rates in blood culture: a cluster randomized trial. Ann Intern Med. 2011; 154:145–51.
8. Schifman RB, Strand CL, Meier FA, Howanitz P]. Blood culture contamination: a College of American Pathologists Q-Probes study involving 640 institutions and 497134 specimens from adult patients. Arch Pathol Lab Med. 1998; 122:216–21.
9. Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman I. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. I Clin Microbiol. 2009; 47:1021–4.

10. Weightman NC, Kerr KG. Blood culture contamination: having your cake and eating it. I Hosp Infect. 2012; 80:101–2.

11. Hopkins K, Huynh S, McNary C, Walker A, Nixon R, Craig—head IE. Reducing blood culture contamination rates: a systematic approach to improving quality of care. Am I Infect Control. 2013; 41:1272–4.

12. Harding AD, Bollinger S. Reducing blood culture contami—nation rates in the emergency department. I Emerg Nurs. 2013; 39:e1–e6.

13. Dawson S. Blood culture contaminants. I Hosp Infect. 2014; 87:1–10.
14. Youssef D, Shams W, Bailey B, O'Neil T], Al-Abbadi MA. Effective strategy for decreasing blood culture contamination rates: the experience of a Veterans Affairs Medical Centre. J Hosp Infect. 2012; 81:288–91.

15. Hall KK, Lyman IA. Updated review of blood culture conta—mination. Clin Microbiol Rev. 2006; 19:788–802.

16. American Academy of Pediatrics. Staphylococcal Infections. In: Pickering LK, Baker CI, Kimberlin DW, Long SS, eds. Red Book®: 2012 Report of the committee on infectious diseases. American Academy of Pediatrics. 2012. 653–68.
17. Kerur B, Salvador A, Arbeter A, Schutzman DL. Neonatal blood cultures: survey of neonatologists' practices. World I Pediatr. 2012; 8:260–2.

18. Weinstein MP. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin Infect Dis. 1996; 23:40–6.

19. Pavlovsky M, Press I, Peled N, Yagupsky P. Blood culture contamination in pediatric patients: young children and young doctors. Pediatr Infect Dis I. 2006; 25:611–4.
Go to : 
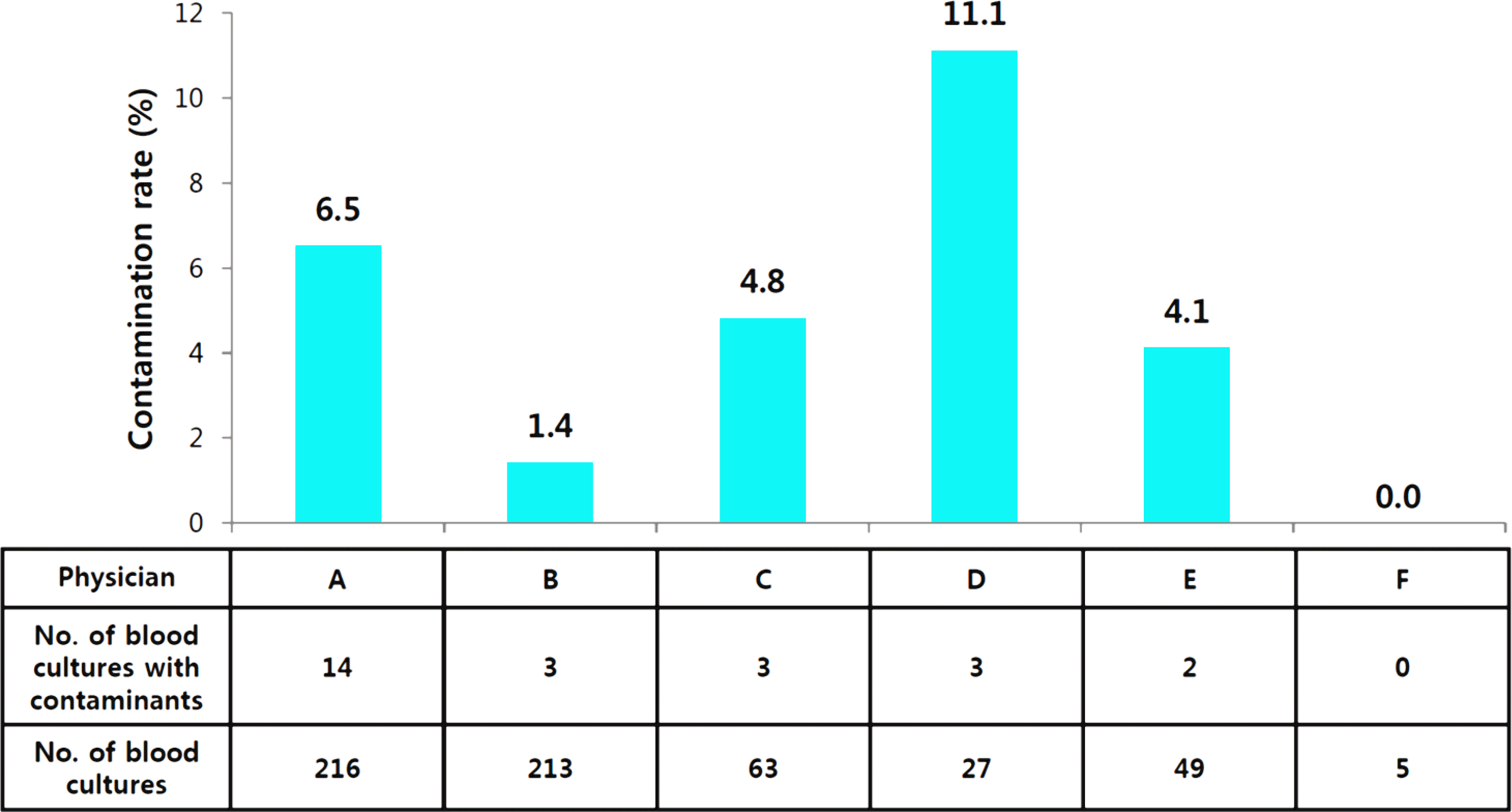 | Fig. 1.Contamination rates in peripheral blood culture by physicians during the period of the real name system. |
Table 1.
Bacteria Considered True Pathogens and Contaminants in Blood Specimens Obtained in a Single Neonatal lntensive Care Unit from March 2013 to February 2014
Table 2.
Rates of Contamination and Bacteremia by Blood Sampling Sites
Table 3.
Comparison of Clinical Factors between Contamination and No Contamination Group in Peripheral Blood Cultures




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download