Introduction
Lemierre syndrome, also known as postanginal septicemia, is a rare disease that often affects healthy adolescents and young adults. In 1936, the French microbiologist Dr. Andre Lemierre described 20 patients with septic thrombophlebitis secondary to pharyngitis and peritonsillar abscess1).
The main causative microorganism of Lemierre syndrome is Fusobacterium necrophorum2). In the pre-antibiotic era, Lemierre syndrome was a complication of head and neck infection, which can cause fulminant disease with septicemia followed by death within 14 days. During the 1960s and 1970s, the syndrome was rarely reported because penicillin was widely prescribed for the treatment of oropharyngeal infection. However, the diagnosis rate for Lemierre syndrome has increased since the 1990s, possibly due to increased antibiotics resistance or change of antibiotics usage patterns, and development of image modalities3).
Considering the risk of systemic septic emboli, it is important to consider this condition very early in examination of individuals with oropharyngeal infection. Physicians should be aware of this disease and consider it as a differential diagnosis in any adolescent patient suffering from pulmonary embolism or neurologic symptoms after oropharyngeal infection.
Case Report
A 16-year-old, previously healthy, male patient visited the emergency department with persistent fever, headache and neck stiffness. He was examined by a pediatrician in the out-patient clinic and was diagnosed with pharyngitis. However, his fever did not subside after four days, and his headache got worse.
His physical examination revealed pharyngeal erythema and neck stiffness. His initial blood pressure was 115/66 mmHg; pulse rate, 96 per minute; respiration rate, 20 per minute; and body temperature, 38.4℃.
His complete blood cell count showed a total white blood cell (WBC) of 16,200 cells/mm3, with a differential count of 90.9% granulocytes. His C-reactive protein (CRP) and erythrocyte sediment rate (ESR) were 36.13 mg/dL and 72 mm/hr, respectively. His initial chest radiograph did not show any infiltration in both lung fields.
Since he complained of severe neck pain and headache with persistent fever, our first consideration was meningitis. Spinal tapping and measurement of the intracranial pressure were performed. The intracranial pressure was 21 cmH2O. The cerebrospinal fluid (CSF) cell differential count revealed a red blood cell (RBC) count of 0/µL; WBC count of 6/µL; CSF protein level of 23.3 mg/dL; and glucose level of 69 mg/dL. Due to the patient's elevated intracranial pressure and WBC count, we could not exclude meningitis as a possible diagnosis. Therefore, parenteral antibiotics were administered to him.
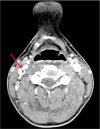
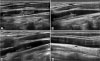
On the second day after admission, he complained of sudden onset of diplopia and limited neck motion. Computed tomography (CT) of the head and neck was performed and it suggested thrombus in the right internal jugular vein (Fig. 1). There were no abnormal findings in the brain parenchyma. In the evening of that day, his diplopia symptom disappeared without any sequelae. On third day after admission, the neck ultrasonography confirmed thrombus in the right internal jugular vein, which was 3.0×0.36 cm (Fig. 2A). On the doppler image, the blood flow of the right internal jugular vein was preserved. We determined this to be a case of Lemierre syndrome with septic thrombophlebitis secondary to oropharyngeal infection. Intravenous treatment with cefotaxime, clindamycin, and metronidazole was initiated.
Fever subsided on the sixth day after admission, which meant he had maintained a ten-day-long fever. On the 10th day of the patient's admission, the thrombus had shrunk to 2.21×0.32 cm (Fig. 2B). On the 17th day, the C-reactive protein had decreased to 0.29 mg/dL. On the 18th day, the neck ultrasonography confirmed shrinkage of the thrombus to 1.03×0.16 cm (Fig. 2C). On the 20th day, the patient was discharged from the hospital with a prescription to continue six days of oral antibiotics.
The results of the blood cultures on the four, six, nine, and 11 days after his admission were all negative. The result of the CSF culture on the day of the patient's admission was also negative. A week after the patient's discharge, neck ultrasonography was performed and finally confirmed resolution of the thrombosis in the right internal jugular vein (Fig. 2D).
Discussion
Lemierre syndrome is a disease characterized by a recent oropharyngeal infection followed by internal jugular vein thrombosis and metastatic emboli to an end-organ such as the brain or lung. It is often caused by anaerobic bacteria, mainly Fusobacterium necrophorum1).
Not all cases of F. necrophorum bacteremia advance to Lemierre syndrome. Some patients with F. necrophorum bacteremia will suffer from mild pharyngitis and then recover with oral antibiotics. However, others with F. necrophorum infection can develop life-threatening diseases such as Lemierre syndrome or peritonsillar abscess3). Even with adequate parenteral antibiotics treatment, this syndrome can follow a fulminant course6).
In the pre-antibiotics era, Lemierre syndrome was a common complication of head and neck infection. After the introduction of antibiotics in the 1940s, it was considered a “forgotten disease”7). Recently, however, an increasing number of journals have been reporting Lemierre syndrome in young adults. According to the review journal by Karkos et al.8), a MEDLINE search, using the term “Lemierre syndrome” as the only keyword, identified six published reports between 1980 and 1990, 50 articles from 1991 to 2000, and 121 article s in the eight years from 2001-2008. Whether this represents a true increase in the incidence of Lemierre syndrome or reflects a higher diagnosis rate due to the introduction of advanced radiologic methods, especially the use of neck computed tomography (CT) scan, remains to be seen8).
According to the article by Ramirez et al.3), authors hypothesized the reason for an increased incidence of Lemierre syndrome is the judicious use of antibiotics after a health campaign about appropriate antibiotic use for upper respiratory infections. As a result, even if the initial diagnosis was uncomplicated pharyngitis, it could progress to Lemierre syndrome. On the other hand, others pointed out the reason for the increased incidence could be due to improved anaerobic blood culture techniques and better identification of Fusobacterium spp9). Considering the high prescription rate of oral antibiotics for upper respiratory infections, we can attribute the higher incidence of Lemierre syndrome in Korea to an increasing number of antibiotics-resistant bacteria.
Approximately 80% of the cases of Lemierre syndrome are caused by anaerobic bacteria called F. necrophorum2). It is a gram-negative bacillus commonly found in the oral cavity, gastrointestinal tract, and female genital tract. F. necrophorum is the most common bacterial agent of pharyngitis in young adults aged 15 to 30 years and can cause persistent sore throat syndrome1011). Therefore, in this age group, clinicians should be more aware of F. necrophorum as a causative organism for pharyngitis than Streptococcus pyogenes11). However, the fact that F. necrophorum is an anaerobe makes it difficult to identify this organism through routine blood culture or a throat culture.
In the case reported herein, all blood cultures were negative and bacteremia was not confirmed. We assumed that this might be due to the fact that blood culture was done on the fourth day after admission. Intravenous antibiotics were started on the first day of admission after CSF tapping. Furthermore, before noticing thrombosis in the internal jugular vein, we could not assume F. necrophorum as a causative organism and anaerobic bacterial blood culture was not included as an initial evaluation.
The pathogenesis of Lemierre syndrome requires several stages. F. necrophorum initially invades the palatine tonsils and the peritonsillar tissue in approximately 87% of cases2). The remaining 13% of cases involve primary pharyngitis, parotitis, sinusitis, mastoiditis, otitis media, and odontogenic infections12). Then the infection spreads to the parapharyngeal space with subsequent invasion of the posterior compartment along the carotid sheath, causing thrombophlebitis of the internal jugular vein. A less common route is direct extension of the thrombophlebitis from the peritonsillar veins2).
Once thrombophlebitis develops in the internal jugular veins, the infection can spread to multiple organs via septic emboli, and lead to metastatic complications. The most frequently involved organs are the lungs and joints. Other sites involved in septic emboli and abscess formation includes muscles, soft tissues, liver, spleen, and central nervous system8). In the case reported herein, the patient did not show any metastatic emboli ot other organs. Although thromboembolism was not detected by brain CT scan, we suspect that the patient's diplopia could be a sign of metastatic emboli in the central nervous system. Had the diplopia persisted, we would have investigated a brain lesion via a brain magnetic resonance image (MRI).
Hypercoagulability usually accompanies Lemierre syndrome, and it plays a major role in the formation of a septic thrombus. The exact mechanism of hypercoagulability is not yet fully understood. In vitro studies of F. necrophorum infection suggested that cell-to-cell contact of virulent strains of F. necrophorum with platelets leads to platelet aggregation. Also, endothelial damage caused by F. necrophorum may be attributed to clot formation in Lemierre syndrome13). However, it is unclear if thrombosis in Lemierre syndrome is attributable to a transient hyperthrombotic state or is a manifestation of thrombophilia triggered by the acute bacterial infection.
According to a study14) of thromboembolic outcomes in Lemierre syndrome, all seven subjects presented thrombophilia at their diagnosis. Three patients were positive for plasma antiphospholipid antibodies, and the other four showed elevated factor VIII activity. Six months later, factor VIII normalized in all cases, and the lupus anticoagulant became negative in two (67%) of three cases. This suggests that thrombophilia is a transient phenomenon caused by an inflammatory process rather than intrinsic hypercoagulability of the patients14).
The main treatment for Lemierre syndrome is parenteral antibiotics. In fact, there is no randomized study to determine optimal agents for the treatment of F. necrophorum infection. Based on several studies, penicillin or clindamycin are not recommended as a monotherapy, because β-lactamase producing strains of F. necrophorum have been reported, and co-infecting pathogens can produce β-lactamase. Additionally, clindamycin is known to have weaker bactericidal activity15).
According to a journal article by Kuppalli et al.16), a combination of ceftriaxone and metronidazole are recommended as they can cover both F. necrophorum and oral streptococci. Another journal17) mentioned that β-lactamase-resistant antibiotics with anaerobic activity are recommended agents, such as ticarcillin-clavulanate, ampicillin-sulbactam, cefoxitin, metronidazole, and amoxicillin-clavulanate. Even though there are differences between these reports, it is certain that clindamycin and penicillin monotherapy should be avoided. Metronidazole is believed to have excellent activity against Fusobacterium spp16). Therefore, we carefully recommend the combination therapy of penicillin/β-lactamase inhibitor or second or third generation cephalosporin with metronidazole. However, the choice of antibiotics can be altered following clinical response.
A typical course of antibiotic therapy ranges from three to six weeks. According to previous reports, the treatment duration can vary from nine to 128 days18). The response to the treatment can be slow, because F. necrophorum is believed to remain viable inside the necrotic tissue for days to a few weeks even after the start of intravenous antibiotic therapy18). Once the clinical symptoms are settled, intravenous treatment can be switched to oral antibiotics. Total treatment duration can be shortened if the clinical progress is favorable. However, less than two weeks of therapy would not be recommended given the fact that relapses have been documented15).
In the case reported herein, we administered the triple antibiotics therapy of cefotaxime, clindamycin, and metronidazole for 19 days, 14 days, and 12 days, respectively. After the patient's discharge, he was prescribed oral third-generation cephalosporin antibiotics for six days and completed three-week antibiotics therapy.
Anticoagulation for treating Lemierre syndrome is still controversial. Some researchers recommend anticoagulation therapy to prevent further progression of thrombus and to decrease the possibility of septic emboli36). Others are concerned with the risk of hemorrhage19). In the case reported herein, the patient was not prescribed anticoagulation therapy. Instead, we performed neck ultrasonography every four to five days to ascertain the status, an eventual shrinkage, of the thrombus.
In conclusion, pediatricians should consider Lemierre syndrome a possible diagnosis if the patient develops severe neck pain with limitation of neck motion, neurologic symptoms, respiratory symptoms that may suggest metastatic thromboemboli after head and neck infection. Also, clinicians should remember F. necrophorum as an important organism causing pharyngotonsillitis in young adults aged 15 to 30 years.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download