Abstract
Purpose
Vaccine evaluation studies were initiated from 2000 by the Ministry of Food and Drug Safety to produce proper data about the safety and immunogenicity of vaccines. The purpose of this study was to review studies and reports on evaluation of vaccine such as immunogenicity, efficacy, effectiveness, safety and other related topics in order to find and analyze the data on the usefulness of each vaccine.
Methods
From 2000 to 2014, the project "The vaccine evaluation" had been performed by several researchers, and studies and reports of vaccine evaluation. We reviewed the results and outcomes of studies regarding the evaluation of vaccine's usefulness and analyzed the possibilities of applying these data for establishing vaccine policies. For each vaccine, data analysis and organization were done according to evaluation fields.
Results
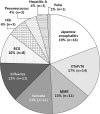
A total of 83 studies were performed on vaccines from 2000 to 2014. For each vaccine, 8 studies were performed on BCG, 14 on DTaP/Td, 1 on poliovirus, 5 on Hib, 3 on pneumococcus, 11 on influenza, 3 on hepatitis A, 11 on MMR, 11 on varicella, and 16 on Japanese encephalitis. All studies were analyzed by the following evaluation area, such as safety, immunogenicity, seroprevalence, persistence of immunity, efficacy, effectiveness, vaccine evaluation methods, quality control product for vaccine, and others.
Figures and Tables
Fig. 1
Numbers and percentage of vaccines performed in vaccine evaluation studies in 2000–2014 (Total number of studies=83).
References
1. Plotkin SL, Plotkin SA, Plotkin SA, Orenstein W. A short history of vaccination. Vaccines. 2004; 5:1–16.

2. Epidemiology and prevention of vaccine-preventable disease. 4th ed. Cheongju: Centers for Disease Control & Prevention;2013.
3. Kang JH. Study to evaluate safety and efficacy of marketed vaccines. Ministry of Food and Drug Safety;2006.
4. Kim HJ, Oh SY, Lee JB. Comparison of each strains (Pasteur, Danish, Tokyo) of BCG efficacy using tuberculin test and adverse reactions. Centers for Disease Control and Prevention;2007.
5. Kim JH. Evaluation for the usefulness of BCG vaccine. Ministry of Food and Drug Safety;2007.
6. Kim HJ. Enhancement of immunogenicity of BCG by genetic manipulation. Centers for Disease Control and Prevention;2007.
7. Kim JH. Evaluation for the usefulness of BCG vaccine. Ministry of Food and Drug Safety;2008.
8. Kim JH. Evaluation for the usefulness of BCG vaccine - a survey for immune status in children with BCG lymphadenitis. Ministry of Food and Drug Safety;2009.
9. Kim JH. Evaluation for the usefulness of BCG vaccine: a survey for immunogenicity of hepatitis B vaccine according to the immunization time of BCG vaccine. Ministry of Food and Drug Safety;2011.
10. Jung JW. Development of alternative identification test for BCG vaccine. Ministry of Food and Drug Safety;2011.
11. Kim YK. Establishment of assay for cell-mediated immunity against tuberculosis and varicella. Centers for Disease Control and Prevention;2014.
12. Kang JH. Age related immunoseroepidemiological study to diphtheria and tetanus among Koreans. Ministry of Food and Drug Safety;2000.
13. Kang JH. Age related immunoseroepidemiological study to tetanus and pertussis in Korean population. Ministry of Food and Drug Safety;2001.
14. Kang JH. Age related seroepidemiological study to pertussis via standardization of pertussis immunoassay among Koreans. Ministry of Food and Drug Safety;2002.
15. Kang JH. The assessment of DTaP vaccine usefulness. Ministry of Food and Drug Safety;2007.
16. Kang JH. The assessment of DTaP vaccine usefulness. Ministry of Food and Drug Safety;2008.
17. kang JH. The assessment of DTaP vaccine usefulness. Ministry of Food and Drug Safety;2009.
18. Kang JH. The seroepidemiologic study of tetanus and pertussis. Ministry of Food and Drug Safety;2011.
19. Han HW. Seroprevalence of diphtheria and tetanus antibodies and considerations on the Td booster vaccination among adults in Republic of Korea. Ministry of Health and Welfare;2002.
20. Choi JH. The assessment of adult Td vaccine usefulness. Ministry of Food and Drug Safety;2007.
21. Choi JH. The assessment of adult Td vaccine usefulness. Ministry of Food and Drug Safety;2008.
22. Baek SY. Manufacture and establishment of national reference of tetanus antitoxin. Ministry of Food and Drug Safety;2010.
23. Jung JW. Standardization of specific toxicity for acellular pertussis vaccine. Ministry of Food and Drug Safety;2011.
24. Kim JG. Study on the establishment of standard material for pertussis toxin and standard pertussis strain. Ministry of Food and Drug Safety;2012.
25. Lee HC. Study on the alternative method for potency test of acellular pertussis vaccines. Ministry of Food and Drug Safety;2014.
26. Hong YJ. Immunogenicity and safety of enhanced inactivated polio vaccine. Ministry of Food and Drug Safety;2010.
27. Kim KH. A study for immunization schedule assessment of Hib vaccine in Korea. Ministry of Food and Drug Safety;2004.
28. Kim KH. Comparison of the schedules for Hib immunization in other countries. Centers for Disease Control and Prevention;2005.
29. Kim KH. A study of strategies development for immunization policy of H. influenzae type b (Hib) vaccine. Centers for Disease Control and Prevention;2007.
30. Baek SY. Study of test for Haemophilus influenzae type b conjugate vaccine by analysis of free saccharide contents. Ministry of Food and Drug Safety;2009.
31. Kim JG. Study on the establishment of reference material for Haemophilus influenzae tybe b polysaccharide. Ministry of Food and Drug Safety;2013.
32. Kim KH. A study of evaluation indexes for antibody production in sera after immunization. Ministry of Food and Drug Safety;2005.
33. Kim KH. A study of analysis method for pneumococcal serotyping and immunogenicity of pneumococcal vaccines. Ministry of Food and Drug Safety;2011.
34. Kim KH. Immunogenicity study of 23-valent pneumococcal polysaccharide vaccine in Korean elderly people. Centers for Disease Control and Prevention;2013.
35. Sung BL. Studies on standardization of genetic stability test of reassortant live influenza vaccine. Ministry of Food and Drug Safety;2006.
36. Lee HJ. Evaluation of efficacy of influenza vaccine in children. Ministry of Food and Drug Safety;2007.
37. Kim WJ. Immunogenicity and safety evaluation of influenza vaccine among adults. Ministry of Food and Drug Safety;2008.
38. Cheong HJ. Guideline for manufacture and quality control of pandemic influenza vaccine. Ministry of Food and Drug Safety;2008.
39. Kim DH. Assessment of persistence of immunity after immunization with influenza vaccine. Ministry of Food and Drug Safety;2009.
40. Ahn C-Y. Development of physicochemical method for quantification of haemagglutinin in influenza vaccine. Ministry of Food and Drug Safety;2010.
41. Kim DH. Evaluation on efficacy and safety of influenza vaccine for children. Ministry of Food and Drug Safety;2011.
42. Sung BL. Development of antigen evaluation system for influenza vaccine. Ministry of Food and Drug Safety;2011.
43. Cheong HJ. Evaluation of immnogenicity of A/H1N1 2009 vaccine. Ministry of Food and Drug Safety;2011.
44. Ahn C-Y. Development of RP-HPLC method for quantitation of haemagglutinin in influenza vaccine (III). Ministry of Food and Drug Safety;2012.
45. Kim DH. Evaluation of field protective efficacy of seasonal influenza vaccine in children, adolescent and elderly. Ministry of Food and Drug Safety;2014.
46. Kim KH. Development and performance of seroepidemiology survey for vaccine preventable diseases. Centers for Disease Control and Prevention;2008.
47. Kim KH. Evaluation of immunogenicity and safety of hepatitis A vaccine in adolescents. Ministry of Food and Drug Safety;2012.
48. Kim JH. Evaluation for the usefulness of hepatitis A vaccine in children. Ministry of Food and Drug Safety;2012.
49. Ki M. Risk analysis of aseptic meningitis after measles, mumps and rubella (MMR) vaccine. Ministry of Food and Drug Safety;2001.
50. Ki M. MMR vaccine effectiveness in elementary school student cohort which had a measles epidemic. Centers for Disease Control and Prevention;2001.
51. Lee GS. An evaluation of school entry immunization certificates in South Korea. Centers for Disease Control and Prevention;2004.
52. Choi BY. Development of goal and strategy of mumps vaccination program. Centers for Disease Control and Prevention;2005.
53. Ki M. Vaccine effectiveness of mumps in Korea. Centers for Disease Control and Prevention;2006.
54. Park HS. Research on the measles vaccination and vaccination policy. Centers for Disease Control and Prevention;2007.
55. Kim KH. Evaluation of efficacy of MMR vaccine. Ministry of Food and Drug Safety;2007.
56. Kim KH. Evaluation of MMR vaccines in Korea. Ministry of Food and Drug Safety;2008.
57. Kim KH. Evaluation of MMR vaccines in Korea - evaluation of immune status of MMR vaccine in different ages. Ministry of Food and Drug Safety;2009.
58. Kim KH. Evaluation of MMR vaccines in Korea - efficacy evaluation of the mumps component of the MMR vaccine. Ministry of Food and Drug Safety;2010.
59. Lee HR. Survey on efficacy and safety of varicella vaccine as a regular vaccine in Korea. Centers for Disease Control and Prevention;2005.
60. Oh SH. Evaluation of effect of varicella vaccination on the incidence and severity of the disease. Centers for Disease Control and Prevention;2006.
61. Oh SH. Evaluation of varicella vaccine utility. Ministry of Food and Drug Safety;2007.
62. Oh SH. Evaluation of varicella vaccine utility - evaluation for immunogenecity and safety of varicella vaccine. Ministry of Food and Drug Safety;2008.
63. Park S. Manufacturing project of a national standard for live varicella vaccine. Ministry of Food and Drug Safety;2008.
64. Kim JO. Establishment of the 2nd national standard for varicella vaccine. Ministry of Food and Drug Safety;2009.
65. Park HS. Evaluation of varicella vaccine effectiveness. Ministry of Food and Drug Safety;2009.
66. Oh SH. Planning research for appraisal of effectiveness of varicella vaccine. Ministry of Food and Drug Safety;2012.
67. Oh HJ. Study on the standardization of virus concentration test for varicella vaccine. Ministry of Food and Drug Safety;2013.
68. Park GY, Ju YR, Choi WY, Choi TK, Cho JH, Nam JH, et al. Studies on the production of antibodies against viral proteins of Japanese encephalitis virus isolated in Korea. National Institue of Health;2002.
69. Cheong HK. A study on the epidemiologic characteristics and control of Japanese encephalitis and rabies. Centers for Disease Control and Prevention;2005.
70. Hong YJ. Immunogenicity and protective efficacy after Japanese encephalitis vaccination. Centers for Disease Control and Prevention;2006.
71. Park CW. Manufacture of Japanese encephalitis vaccine reference for potency test. Ministry of Food and Drug Safety;2006.
72. Hong YJ. The assessment of Japanese encephalitis vaccine usefulness. Ministry of Food and Drug Safety;2007.
73. Hong YJ. Adverse reaction of Japanese encephalitis vaccine. Centers for disease Control and Prevention;2007.
74. Hong YJ. The assessment of Japanese encephalitis vaccine usefulness. Ministry of Food and Drug Safety;2008.
75. Hong YJ. The assessment of Japanese encephalitis vaccine effectiveness. Ministry of Food and Drug Safety;2009.
76. Hong YJ. Analysis of the current development status and national policies regarding JE vaccine. Centers for Disease Control and Prevention;2009.
77. Kim JO. Construction of recombinant Japanese encephalitis virus (JEV) E antigen and neutralizing JEV E monoclonal antibodies. Ministry of Food and Drug Safety;2009.
78. Nam JH. Development of in vitro potency assay of antigen titer for Japanese encephalitis vaccine using monoclonal antibody. Ministry of Food and Drug Safety;2010.
79. Hong YJ. The assessment of Japanese encephalitis vaccine usefulness. Ministry of Food and Drug Safety;2010.
80. Kim JO. Establishment of in vitro potency assay for Japanese encephalitis (inactivated) vaccine - Collaborative study on quantitative assay for Japanese encephalitis virus (JEV) antigen using neutralizing JEV monoclonal antibodies. Ministry of Food and Drug Safety;2011.
81. Hong YJ. Neutralizing antibody titer above 15 years old in Korea. Ministry of Food and Drug Safety;2012.
82. Ahn C-Y. Study of neutralization assay for Japanese encephalitis. Ministry of Food and Drug Safety;2012.
83. Oh HJ. The collaborative study on establishment of national standard (3rd) and potency assay for Japanese encephalitis vaccine. Ministry of Food and Drug Safety;2014.
84. The Korean Pediatric Society. Immunization recommendation for children and adolescents. In : Kim KH, editor. Immunization guideline. 8th ed. Seoul: The Korean Pediatric Society;2015. p. 2–3.
85. Yeo MJ, Choi KH, Kang SH, Kim SH, Lee SY, Song MH, et al. Japanese encephalitis in Korea, 2010. Case reports and epidemiology. J Korean Neurol Assoc. 2011; 29:335–338.
86. Yun KW, Lee HJ, Kang JH, Eun BW, Kim YJ, Kim KH, et al. Safety and immunogenicity of a freeze-dried, Vero cell culturederived, inactivated Japanese encephalitis vaccine (KD-287, ENCEVAC®) versus a mouse brain-derived inactivated Japanese encephalitis vaccine in children: a phase III, multicenter, double-blinded, randomized trial. BMC Infect Dis. 2015; 15:7.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download