Abstract
Infective endocarditis (IE) caused by methicillin-resistant Staphylococcus aureus (MRSA) has become a worldwide concern. We present a case of a 12-year-old child with IE of the native mitral valve due to MRSA infection after an invasive dental procedure. Based on the clinical symptoms and the presence of cerebrospinal fluid pleocytosis, the patient was initially diagnosed with presumed bacterial meningitis and treated with empiric antibiotics. On the third day of hospitalization, MRSA was cultured from the initial blood samples and vegetation was observed on the mitral valve during an echocardiogram, findings which are compatible with a diagnosis of IE. The revised guidelines for antibiotic prophylaxis for the prevention of IE advise that IE prophylaxis for dental procedures is reasonable only for patients with underlying cardiac conditions, who are at the highest risk of adverse outcomes from IE. However, in this case, the patient had no high risk factors indicative of IE prophylaxis, except for mitral valve prolapse. She had no recurrence of IE over a follow-up period of 12 months.
Infective endocarditis (IE) is an infection of the heart valves, large intrathoracic vessels, and intracardiac foreign bodies. Despite improvements in the diagnosis and treatment of IE, the in-hospital mortality and morbidity remains high12). Neurologic complications appear in 20% to 40% of patients with left-side IE, with ischemic stroke being the most common (50%), followed by cerebral hemorrhage (20%), and other complications such as transient ischemic attack, meningitis, infectious aneurysm, and brain abscess (30%)3). The best predictor of these neurologic complications is delayed initiation of antibiotic therapy4).
The true incidence of IE among otherwise healthy children is unclear. Incidence of IE in children with structurally normal hearts and no predisposing factors has been reported from 19% to 33% 567). Previous study showed that IE in this population tended to be community-acquired left-sided infections caused by traditional “community” pathogens, including Staphylococcus aureus, Streptococcus pneumoniae, Kingella kingae, and Haemophilus species7).
The incidence of IE caused by S. aureus, especially methicillin-resistant Staphylococcus aureus (MRSA) is increasing. In fact, S. aureus has become the leading cause of IE in Western Europe and North America89). It is responsible for the highest rates of morbidity and mortality910). Similarly, in Korea, Choi et al.11) reported that the most common causative organism in pediatric IE was S. aureus, which was found in 14 of 29 cases (48.3%), out of which three cases were MRSA. Here, we report a case of 12-year-old girl who initially presented with presumed bacterial meningitis but was later diagnosed as IE due to MRSA probably associated with previous invasive dental procedure.
A previously healthy 12-year-old girl was admitted with a 4-day history of high fever, along with a 2-day history of headache and a 1-day history of vomiting. She had received treatment of the gingiva, including braces in a dental clinic 2 weeks earlier. On the day of admission, body temperature was 38.4℃, heart rate was 96 beats per minute, and blood pressure was 110/70 mm Hg. Breathing sounds were clear without crackles and there was no cardiac murmur. The chest radiograph was unremarkable, with normal appearance of the mediastinum and no infiltrates. There was no wound of skin or mucous membrane.
The initial laboratory results were as follows: hemoglobin 12.4 g/dL, white blood cell (WBC) count 7,300/µL (polymorphonuclear leukocytes 93.0%, lymphocytes 3.1%, monocytes, 2.7%) platelet count 81×103/µL, C-reactive protein (CRP) 10.75 mg/dL (normal range, <0.5 mg/dL), sodium 136 mEq/L, aspartate aminotransferase 70 U/L, alanine aminotransferase 54 U/L, and procalcitonin 2.35 ng/dL (normal range, <0.5 ng/dL). The patient's prothrombin time was 13.7 seconds (normal values, 11.6 to 15.5 seconds), international normalized ratio was 1.03, and activated partial thromboplastin time was 46.3 seconds (normal values, 28.0 to 45.0 seconds). Urine analysis revealed microscopic hematuria (urine red blood cell >30/high power field). She complained of headache with photophobia and lower leg weakness. Her motor strengths were decreased to grade III in both lower extremities, while her sensory response was intact. She showed neck stiffness and cerebrospinal fluid (CSF) analysis was performed to rule out meningitis. The results revealed the following: WBC 160/µL (neutrophils 20%, lymphocytes 80%), glucose 73 mg/dL, total protein 40.2 mg/dL. Consequently, she received ceftriaxone (75 mg/kg/day) and vancomycin (60 mg/kg/day) as empiric therapy for presumed bacterial meningitis.
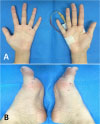
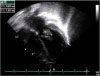
On the third day of hospitalization, she had persistent high fever and showed painless hemorrhagic cutaneous lesions on the soles and palms (Fig. 1). Blood culture reports revealed growth of MRSA which is susceptible to clindamycin, gentamicin, and vancomycin but is resistant to oxacillin. CSF culture revealed no growth on the same day. The cardiac murmur became audible on examination and the level of N-terminal fragment of B-type natriuretic peptide was elevated to 280.5 pg/mL. On day 4 of hospitalization, echocardiography showed mitral valve prolapse with a large oval-shaped oscillating mass (16.5×6.9 mm) attached to the anterior leaflet of the mitral valve (Fig. 2). The diagnosis of IE was made at this point, and treatment with vancomycin was continued along with the addition of amikacin (20 mg/kg/day). Left ventricular systolic contracti lity was preserved, but diastolic dysfunction was observed on Doppler echocardiogram. Tissue Doppler image revealed increased left ventricular filling pressure, which is compatible to the grade 2 diastolic dysfunction (pseudonormalized pattern). Abdominal computed tomography was performed to rule out peripheral embolism; no embolic lesion was revealed.
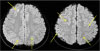
On day 5 of hospitalization, after removing her braces, brain magnetic resonance imaging (MRI) was performed to rule out cerebral embolic lesions. It revealed multifocal disseminated cerebritis and a few scattered small infarcts in the right frontal and the left parietal cortical areas (Fig. 3). However, there were no findings of seizures or focal neurologic signs in this patient.
On day 8 of hospitalization, follow-up echocardiography showed decreased vegetation size (7.1×4.1 mm) with normal left ventricular systolic function (fractional shortening 36.3%, ejection fraction 66.4%). However, the fever persisted and repeated blood culture showed isolation of MRSA until day 9 of hospitalization. The trough level of vancomycin was 10.4 µg/mL (normal range, 10 to 15 µg/mL). Persistent fever and demonstration of bacteremia for more than 8 days despite adequate antimicrobial therapy are considered indications for valve surgery. Therefore, we consulted a cardiothoracic surgeon about the necessity for operation. However, the next day, the CRP level was decreased to 5.5 mg/dL and the size of the vegetation had shrunk to less than half its original size. Hence, we continued the antibiotic treatment with close monitoring of the vital signs. Finally, repeated blood culture showed no growth on day 11 of admission and her fever gradually subsided thereafter.
The follow-up laboratory results on day 16 of hospitalization were as follows: WBC count 4,340/µL (polymorphonuclear leukocytes 65.9%, lymphocytes 17.7%, monocytes 9.8%), hemoglobin 10.1 g/dL, the platelets 402×103/µL, and CRP 2.71 mg/dL. The follow-up serum trough level of vancomycin was elevated to 20.2 µg/mL on hospital day 19, and the dosage was reduced to 10 mg/kg/dose every 6 hours. The 20 mg/kg/day amikacin dosage was maintained until hospital day 21. On hospita l day 21, echocardiography revealed disappearance of the vegetation with residual mitral insufficiency and mitral valve prolapse.
On hospital day 26, the CRP level was decreased to 0.48 mg/dL and serial blood cultures did not identify any recurrence of MRSA. According to the dose adjustment, vancomycin trough level was 10.3 µg/mL on hospital day 36. Intravenous treatment of vancomycin was continued until hospital day 46. There was no evidence of nephrotoxicity in the laboratory results.
Follow-up brain MRI at 6 months showed disappearance of the previously seen multifocal small high signal intensity nodular lesions at the left basal ganglia and bilateral frontoparietal white matter area. Currently, the patient has been treated by enalapril and out-patient follow-up care for 12 months with no recurrence of vegetation on echocardiograms
IE is a life threatening bacterial infection. Neurologic complications such as meningitis, stroke, cerebral hemorrhage, and cerebral abscess are frequent and increase the risk of morbidity and mortality. Despite majo r developments in the field of medicine, the diagnosis, management, and prediction of IE remain challenging. The average diagnostic delay of IE is reported to be approximately 30 days12). Most vegetations occur in areas where there is a pressure gradient with resulting turbulence of blood flow. In the past, patients with congenital heart lesions in which blood is ejected at a high velocity through a hole or stenotic orifice were the most susceptible to endocarditis13).
Although early reports described IE exclusively in children with structurally abnormal hearts due to either congenital heart disease of acquired rheumatic heart disease, this infection has more recently been reported in diverse groups of patients7). The complexities of patient management in neonatal and pediatric intensive care units have increased the risk of IE in children with structurally normal hearts and with any other readily identifiable risk factors. In these situations, the infection usually involves the aortic or mitral valve secondary to S. aureus bacteremia1314). Recently, body piercing constitutes a high risk procedure due to the possible acquisition of IE6). In the present case, the patient had no ear piercing and no tampon use before. Interestingly, the source of infection, braces and an invasive dental procedure causing mucosal bleeding could be traced, and the patient had no high risk factors of IE prophylaxis except mitral valve prolapse. Mitral valve prolapse with mitral regurgitation was still observed on follow-up echocardiogram in this patient.
Mitral valve prolapse was a controversial issue in the past. One report suggested that mitral valve prolapse is an important underlying defect in pediatric patients with endocarditis15). However, the revised guidelines for antibiotic prophylaxis for the prevention of IE were released in 2007 by the American Heart Association and the American College of Cardiology16); the primary reason for the revision of the IE prophylaxis guidelines was that the risk of antibiotic-associated adverse events was considered to exceed the benefits, if any, from prophylactic antibiotic therapy. In addition, good oral, dental, and skin hygiene are recommended to reduce the risk. Especially, dental hygiene is of major importance for the prevention of IE. The committee concluded that only an extremely small number of cases of IE might be prevented by antibiotic prophylaxis for dental procedures even if such prophylactic therapy was 100% effective. Therefore, mitral valve prolapse no longer warrants antibiotic prophylaxis for any denta l conditions according to the revised guideline.
Dental procedures are a frequent source of bacteremia, particularly from viridans group Streptococci. Besides viridians group Streptococci, other bacterial species such as S. aureus and coagulase negative staphylococci were also isolated from blood cultures following dental operative procedures in children17). In contrast, S. aureus that was identified in this case tended to prevail, identifying the skin flora as a major infection source. S. aureus colonizes the skin and mucous membranes of 30% to 50% of healthy adults and children. Nasal, skin, vaginal, and rectal carriage are the primary reservoirs for S. aureus . Rates of carriage of more than 50% occur in children with desquamating skin disorders or burns and in people with frequent needle use18). Nasal swab culture was not performed in the present case because the patient had no previous history of recurrent skin infection. However, it will be necessary to rule out the carriage state in this patient if any recurrence of infection due to S. aureus is happening.
MRSA has been endemic in most United States hospitals since the 1980s, recently accounting for more than 60% of health care-associated S. aureus infections in intensive care units reported to the Centers for Disease Control and Prevention18). Health care-associated IE was defined as either nosocomial infection or non-nosocomial health care-associated infection. In this case, the patient had no past history of admission or recent antibiotic treatment. Presumed place of acquisition of MRSA in this patient is not clear. After the dental procedure, she touched the gingiva with her hands several times due to discomfort of her braces at home. The most frequent manifestation of community-associated MRSA infections is skin and soft tissue infection, but invasive disease also occurs
In fact, in accordance with the updated guidelines, antibiotic prophylaxis is now being restricted to only a small number of cardiac conditions with very high risk of adverse outcomes from IE. However, a recent study showed that the IE incidence has increased in the United States over the past decade19). With regard to the microbiology of IE, there has been a significant rise in the incidence of Streptococcus IE cases since the 2007 guideline revisions. In addition, the proportion of IE due to Staphylococcus increased from 33% in 2000 to 40% in 2011, a relative increase of 18.9%. In Korea, there are no data on the IE trends since this major practice guideline change.
In the present case, the total duration of vancomycin and amikacin administration were 46 and 21 days, respectively. There are no randomized controlled trials comparing vancomycin with a combination of vancomycin and an aminoglycoside. The addition of gentamicin for the first 3 to 5 days is optional but can be hypothesized to accelerate the killing of Staphylococci; this concept is based on extrapolation from an experimental model14). On the other hand, gentamicin is no longer recommended for staphylococcal IE involving a native valve, because there is no documented clinical benefit, as well a risk of nephrotoxicity20). However, the patients in the above-mentioned study were exclusively adults with underlying diseases who were taking concomitant nephrotoxic drugs such as angiotensinconverting enzyme inhibitors, angiotensin receptor blockers, or nonsteroidal anti-inflammatory drugs. Therefore, the results from studies of medical management in adults with IE could not be directly extrapolated to children with IE. Up to now, intravenous vancomycin added gentamicin is recommended for treatment of bacteremia due to MRSA for life-threatening infections in pediatric patients18).
Cerebral complications are the most frequent and severe extracardiac complications of IE. Systematic MRI of the brain shows cerebral abnormalities in up to 80% of patients, with asymptomatic embolic events being the most common (50%)21). The patient in this case showed evidence of native mitral valve IE complicated by silent cerebral embolism and meningitis. Vegetation that are large, mobile, or in the mitral position, as well as IE due to S. aureus are associated with an increased risk of symptomatic embolism. Nevertheless, the role of the vegetation size as the only indication for surgery is far from clear. In this case, the patient showed a bulky vegetation over 10 mm in size on the mitral valve. However, she recovered completely with antibiotic treatment without valve surgery. We speculate that this outcome was probably due to the early initiation of antimicrobial therapy before the confirmatory diagnosis of IE. The risk of central nervous system embolic events in IE decreases dramatically after the initiation o f effective antimicrobial therapy, to less than 1.71/1,000 patient days in the second week4).
S. aureus meningitis is a challenging disease and little is known about its epidemiology. There are no established management guidelines. The most commonly used antimicrobial regimens for S. aureus meningitis have been nafcillin for methicillin-susceptible S. aureus strains and vancomycin with or without rifampin or trimethoprim/sulfamethoxazole for MRSA. The incidence of MRSA meningitis increased over the last 5 years of the previous study22).
In summary, in this case, the patient initially showed signs of meningeal irritation and CSF pleocytosis. However, it is unclear whether the meningitis was just a coincidental finding, because the presence of pathogen in the CSF is very transient in most cases of S. aureus meningitis23). Primary health care providers must consider the possibility of IE while evaluating children who present with features of meningitis combined with septicemia.
Figures and Tables
References
2. Gunebakmaz O, Kaya MG, Kaya EG, Ardic I, Yarlioglues M, Dogdu O, et al. Mean platelet volume predicts embolic complications and prognosis in infective endocarditis. Int J Infect Dis. 2010; 14:e982–e985.

3. San Roman JA, Vilacosta I, Lopez J, Sarria C. Critical questions about left-sided infective endocarditis. J Am Coll Cardiol. 2015; 66:1068–1076.

4. Dickerman SA, Abrutyn E, Barsic B, Bouza E, Cecchi E, Moreno A, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J. 2007; 154:1086–1094.

5. Coward K, Tucker N, Darville T. Infective endocarditis in Arkansan children from 1990 through 2002. Pediatr Infect Dis J. 2003; 22:1048–1052.

6. Kovarik A, Setina M, Sulda M, Pazderkova P, Mokracek A. Infective endocarditis of the tricuspid valve caused by Staphylococcus aureus after ear piercing. Scand J Infect Dis. 2007; 39:266–268.

7. Marom D, Ashkenazi S, Samra Z, Birk E. Infective endocarditis in previously healthy children with structurally normal hearts. Pediatr Cardiol. 2013; 34:1415–1421.

8. Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005; 293:3012–3021.
9. Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med. 2013; 368:1425–1433.
10. Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012; 54:1230–1239.

11. Choi EN, Kwon JH, Choi KM, Hwang HD, Sin KM, Choi JY, et al. A clinical study of infective endocarditis in childhood. Korean J Pediatr. 2004; 47:844–850.
12. Knudsen JB, Fuursted K, Petersen E, Wierup P, Molgaard H, Poulsen SH, et al. Infective endocarditis: a continuous challenge. The recent experience of a European tertiary center. J Heart Valve Dis. 2009; 18:386–394.
13. Saiman L, Prince A, Gersony WM. Pediatric infective endocarditis in the modern era. J Pediatr. 1993; 122:847–853.

14. Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, et al. Infective endocarditis in childhood: 2015 update: a scientific statement from the American Heart Association. Circulation. 2015; 132:1487–1515.
15. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol. 1991; 68:90–94.

16. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116:1736–1754.
17. Lucas VS, Omar J, Vieira A, Roberts GJ. The relationship between odontogenic bacteraemia and orthodontic treatment procedures. Eur J Orthod. 2002; 24:293–301.

18. American Academy of Pediatrics Committee on Infectious Diseases. Staphylococcal infections. In : Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red book: 2012 report of the committee on infectious diseases. 29th ed. EIK Groove Village: American Academy of Pediatrics;2012. p. 653–667.
19. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015; 65:2070–2076.

20. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015; 132:1435–1486.

21. Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med. 2010; 152:497–504.

22. Aguilar J, Urday-Cornejo V, Donabedian S, Perri M, Tibbetts R, Zervos M. Staphylococcus aureus meningitis: case series and literature review. Medicine (Baltimore). 2010; 89:117–125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download