Abstract
Purpose
Bacillus cereus has been reported as the cause of nosocomial infections in cancer patients. In our pediatric cancer ward, a sudden rise in the number of patients with B. cereus bacteremia was observed in 2013 to 2014. This study was performed to investigate the molecular epidemiology of increased B. cereus bacteremia cases in our center.
Methods
Pediatric cancer patients who developed B. cereus bacteremia were identified from January 2001 to June 2014. The B. cereus bacteremia in this study was defined as a case in which at least one B. cereus identified in blood cultures, regardless of true bacteremia. Available isolates were further tested by multilocus sequence typing (MLST) analysis. A retrospective chart review was performed.
Results
Nineteen patients developed B. cereus bacteremia during the study period. However, in 2013, a sudden increase in the number of patients with B. cereus bacteremia was observed. In addition, three patients developed B. cereus bacteremia within 1 week in July and the other three patients within 1 week in October, respectively, during emergency room renovation. However, MLST analysis revealed different sequence types without consistent patterns. Before 2013, five tested isolates were ST18, ST26, ST177, and ST147-like type, and ST219-like type. Isolates from 2013 were ST18, ST73, ST90, ST427, ST784, ST34-like type, and ST130-like type.
Bacillus cereus is a gram-positive, spore-forming rod. Endospores from Bacillus species are resistant to advers environmental conditions, which enable B. cereus to be ubiquitous in nature1). B. cereus is a major bacterium that causes food poisoning23). In addition, this species has been reported as a cause of nosocomial infection, especially in immunocompromised patients4567891011), and several nosocomial outbreaks of B. cereus have been reported4121314). We experienced a sudden increase of B. cereus bacteremia episodes in 2013. More importantly, there were two clusters of B. cereus bacteremia episodes in July and October, where three patients developed bacteremia within 1 week in July and another three patients also had bacteremia within 1 week in October. This observation prompted us to investigate the molecular epidemiology of B. cereus bacteremia that occurred in our pediatric cancer center and to examine whether the bacteremia episodes were linked by a common strain.
We retrospectively reviewed the medical records of pediatric cancer patients who developed B. cereus bacteremia between January 2001 and June 2014. The Institutional Review Board of Samsung Medical Center (IRB File No. 2015-04-031) approved this study.
The B. cereus bacteremia in this study was defined as a case in which at least one B. cereus identified in blood cultures, regardless of true bacteremia. As regards that all patients in this study had central venous catheters, catheter-related bloodstream infection (CRBSI) was defined when growth of microbes from blood drawn from a catheter hub at least 2 hours before microbial growth is detected in blood samples obtained from a peripheral vein. The true bacteremia was defined when both one peripheral blood culture and every catheter blood cultures, are positive for B. cereus without time-to-positivity. The contamination or colonization were defined if it did not met the criteria of CRBSI or true bacteremia.
The isolates of B. cereus bacteremia obtained in blood cultures, which had been stored in a freezer, were subcultured by inoculating them on blood agar plates and incubating at 37℃ for 18 hours.
A loopful of isolates of each B. cereus strain was suspended in phosphate-buffered saline and centrifuged for 10 minutes at 7,500 rpm. After removing the supernatant, the pellet was suspended in 480 µL of 50 mM ethylenediaminetetraacetic acid, and 60 µL 10 mg/mL lysozyme and 60 µL of 10 mg/mL lysostaphin (Sigma-Aldrich, St. Louis, MO, USA) were added. The solution was incubated for 30 minutes at 37℃. Next, extraction of genomic DNA from B. cereus was performed using the QIAamp DNA mini kit (Qiagen, Hilden, Germany).
Seven genes chosen for multilocus sequence typing (MLST) analysis included glpF (glycerol uptake facilitator protein), gmk (guanylate kinase, putative), ilvD (dihydroxy-acid dehydratase), pta (phosphate acetyltransferase), pur (phosphoribosylaminoimidazolecarbo xamide), pycA (pyruvate carboxylase), and tpi (triosephosphate isomerase) (http://pubmlst.org).
The polymerase chain reaction (PCR) conditions used for amplification were as follows. A PCR was performed for 35 cycles with a 25 µL PCR mixture containing each deoxynucleoside triphosphate at a concentration of 10 mM, each primer at a concentration of 40 pmol, 500 ng of genomic DNA, and 1 U of Taq polymerase. An initial denaturation at 94℃ for 5 minutes was followed by 94℃ for 30 seconds. The primers and annealing temperatures for each primer set are shown in Table 1. The annealing time was 30 seconds, which was followed by extension for 30 seconds at 72℃. After 35 cycles, the reaction was completed by a final extension at 72℃ for 7 minutes. Nucleotide sequencing data was obtained.
A total of 20 episodes of B. cereus bacteremia occurred in 19 patients during the study period. One patient had two episodes of B. cereus bacteremia in September 2009 and October 2013, respectively. Eleven episodes (11/20, 55%) occurred between January 2013 and June 2014 (Fig. 1). The characteristics of study patients are shown in Table 2. Bacteremia in three patients (isolates 10, 11, and 12) were clustered within 1 week in July 2013 and in another three patients (isolates 14, 15, and 16) were clustered within 1 week in October 2013.
In the cluster cases of July 2013, blood cultures from two patients were collected in an emergency room (ER; isolates 10 and 12) and one patient (isolate 11) developed bacteremia while staying in the pediatric cancer ward. The patient with isolate 10 underwent a second autologous peripheral blood stem cell transplantation for medulloblastoma and the patient with isolate 12 had glioma. Both patients developed fever during radiotherapy and were diagnosed to have CRBSI caused by B. cereus. Of note, the patient with isolate 12 continued to have positive culture results from the second blood culture, which was collected in the pediatric cancer ward. The patient with isolate 11 developed bacteremia in the cancer ward during the induction treatment for leukemia.
In the cluster cases of October 2013, blood cultures from two patients were collected in the ER (isolates 14 and 16) and one patient (isolate 15) developed bacteremia while staying in the pediatric cancer ward. The patient with isolate 14 visited the ER with neutropenic fever and blood cultures grew B. cereus only from the third lumen of a Hickman catheter. The follow-up blood cultures collected in the cancer ward before initiation of appropriate antibiotic therapy showed no further bacteremia in any Hickman catheter lumens. The patient with isolate 16 visited the ER with neutropenic fever and the blood cultures grewB . cereus only from a peripherally inserted central catheter. Meanwhile, the case with isolate 15 developed CRBSI in the transplantation unit, where the patient was undergoing peripheral blood stem cell collection for transplantation.
In the cluster cases of July 2013, all three cases of B. cereus infection appeared to be true bacteremia. However, in the cluster cases of October 2013, two cases (isolates 14 and 16) appeared to be contamination or colonization, and only one case with isolate 15 appeared to be true bacteremia. All six patients had a central catheter. Three of the patients were in a neutropenic state. In relation to stem cell transplantation, one patient was 113 days post second autologous peripheral blood stem cell transplantation. Co-infection was observed in one patient (isolate 11) with parainfluenza infection.
Overall mortality at 28 days from B. cereus bacteremia onset was 10.5% (2/19). One patient died of suspected septic shock without any identified microorganism while the other patient died of Enterobacter aerogenes sepsis.
Of the 20 episodes of B. cereus bacteremia, 15 isolates were available for MLST analysis. The analysis showed 12 sequence types (ST18, ST24, ST26, ST34-like type, ST73, ST90, ST130-like type, ST147-like type, ST177, ST219-like type, ST427, and ST784) (Tables 3, 4). The phylogenetic relationship of the 15 isolates according to MLST analysis using seven genes concatenately is shown in Fig. 2. All available five isolates from the July 2013 and October 2013 clusters had different STs, respectively (Table 4).
There are three STs that were identified in two or more isolates. ST18 was identified in isolate 7 (year 2011) and 12 (year 2013). ST73 was identified in isolates 10 (year 2013) and 20 (year 2014). ST177 was observed in isolates 5 (year 2009) and 19 (year 2014). Even though these isolates share the same STs, there were no epidemiological links. Except for these three STs, there was no common ST among the rest of the 9 isolates (Fig. 2).
B. cereus is well known as a common cause of bacteremia in immunocompromised patients4567891011) and nosocomial infection has been reported in previous studies4121314). In this study, there was also a suspicion for nosocomial infection of B. cereus bacteremia in our pediatric cancer center because of a sudden increase of B. cereus bacteremia episodes in 2013 to 2014. However, further investigation for molecular epidemiology by MLST revealed little possibility of nosocomial transmission of a single strain of bacteria.
Nosocomial infection of B. cereus is well known. Outbreaks that originated from common contaminant sources, such as linens and towels, were reported in some studies121314). In those cases, similarity of B. cereus on MLST or pulsed-field gel electrophoresis analysis was reported between the B. cereus isolated from patient blood cultures and environment cultures. Since B. cereus endospores are known to be resistant to heat and alcohol118), it was thought that endospores could have survived in the hospital environment, such as washing machines or hands, to cause the outbreak of B. cereus bacteremia. In those studies, control measures against the outbreak were implemented. It included sterilizing linens by autoclaving, cleansing washing machines with a powerful detergent, and washing hands with soap instead of alcohol19), and promoting the use of gloves among the hospital staff during patient care. After implementation, the incidence of B. cereus nosocomial infection was decreased significantly1213).
On the contrary, pseudo-outbreaks are also possible in a situation where an organism is recovered from culture at a higher rate than usual and that cannot be clinically correlated with the supposed infection2021). This situation may result from systemic extrinsic contamination during specimen collection or processing or intrinsic contamination at the time the culture medium was manufactured or prepared2122).
In this study, the suspicion for outbreak caused by B. cereus from a common source began when we observed clusters of B. cereus bacteremia in three patients in 1 week in July and October, 2013. However, MLST analysis revealed a total of 12 different STs in 15 isolate s (Table 3). In 12 STs, ST18, ST73, ST90, and ST147 were reported previously in human bacteremia cases (http://pubmlst.org)232425). The ST24, ST34, ST177, and ST427 types are known to be found in food, feces, and the human body (http://pubmlst.org; http://cdc.go.kr)2526). The remaining ST26, ST130, ST219, and ST784 types were reported in soil (http://mlstoslo.uio.no). Therefore, it appears that the possibility of a common infection source for B. cereus bacteremia was low in the current study.
Upon additional investigation, we found that a part of the ER was under remodeling construction between July 1 2013 and October 10 2013. Among a total of six cases of B. cereus bacteremia in July and October 2013, four cases of blood cultures were collected in the ER. The increase of B. cereus bacteremia following hospital construction was previously reported. Loeb et al.27) reported pseudo-bacteremia, speculating that Bacillus species acting as airborne contaminants from the construction site likely seeded the plastic lids of the stored blood culture bottles. Another Bacillus outbreak after renovation work was reported by Ohsaki et al.21). They considered the case as an undetected Bacillus pseudo-outbreak after the hospital renovation work. They also considered that filters of the heating, ventilation, and air-conditioning systems and towels and gowns were probable sources of the outbreak21). However, we did not discover a definite correlation between ER renovation and the increase of B. cereus bacteremia.
Although there is a possibility that increased B. cereus bacteremia in our center were a part of a pseudooutbreak, we could not prove it. It appears that the increase of B. cereus bacteremia came from temporary contamination with diverse B. cereus STs, which were distributed throughout various environments rather than from a common environmental source contaminated with a specific B. cereus ST.
Our study has several limitations. First, this study was performed retrospectively. We could not perform the environmental cultures at the time every B. cereus bacteremia case developed. We did not obtain a questionnaire from the patients and their parents to find a common exposure source such as food, specific behavior, and contact history to the person or materials. Second, except MLST analysis, additional studies, such as virulence gene analysis or antibiotic susceptibility tests, were not performed on these B. cereus isolates. Finally, because of the small sample number limited to pediatric cancer patients, we could not identify the risk factors for B. cereus bacteremia and the difference of clinical course according to B. cereus sequence type.
In conclusion, although a sudden increase in B. cereus bacteremia cases was observed in our pediatric cancer center, there was no significant evidence suggesting a true outbreak caused by a single ST based on molecular epidemiological links. However, there may be a possibility of a pseudo-outbreak caused by multiple STs in the process of the renovation work of the hospital. Continuous monitoring is needed and hospital environmental culture surveillance is required especially when increased B. cereus bacteremia cases are observed or possible environmental contamination is suspected. Furthermore, the intensified implementation of control measures should be maintained according to the comprehensive and effective infection control policy.
Figures and Tables
Fig. 1
Distribution of Bacillus cereus bacteremia episodes by year. Number in the bar indicates each bacteremia episode. No. 5 and 14 are two episodes from the same patient.

Fig. 2
Phylogenetic tree of 15 tested isolates. Numbers in parentheses indicate each isolate ID. Isolates from episodes 1, 2, 3, 4, and 16: not available. The scale at the bottom represents genetic distances in nucleotide substitutions per site.
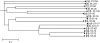
Table 1
Primers Used for Polymerase Chain Reaction Amplification and Sequencing of Bacillus cereus Group Housekeeping Genes (http://pubmlst.org)

Table 3
Results of Seven Genes and Sequence Types of Each Isolates

Abbreviations: ID, identification; glp, glycerol uptake facilitator protein; gmk, guanylate kinase, putative; ilv, dihydroxy-acid dehydratase; pta, phosphate acetyltransferase; pur, phosphoribosylaminoimidazolecarboxamide; pyc, pyruvate carboxylase; tpi, triosephosphate isomerase; ST, multilocus sequence type.
References
2. Pirttijarvi TS, Andersson MA, Scoging AC, Salkinoja-Salonen MS. Evaluation of methods for recognising strains of the Bacillus cereus group with food poisoning potential among industrial and environmental contaminants. Syst Appl Microbiol. 1999; 22:133–144.

3. Slaten DD, Oropeza RI, Werner SB. An outbreak of Bacillus cereus food poisoning: are caterers supervised sufficiently. Public Health Rep. 1992; 107:477–480.
4. Ozkocaman V, Ozcelik T, Ali R, Ozkalemkas F, Ozkan A, Ozakin C, et al. Bacillus spp. among hospitalized patients with haematological malignancies: clinical features, epidemics and outcomes. J Hosp Infect. 2006; 64:169–176.

5. Akiyama N, Mitani K, Tanaka Y, Hanazono Y, Motoi N, Zarkovic M, et al. Fulminant septicemic syndrome of Bacillus cereus in a leukemic patient. Intern Med. 1997; 36:221–226.

6. Arnaout MK, Tamburro RF, Bodner SM, Sandlund JT, Rivera GK, Pui CH, et al. Bacillus cereus causing fulminant sepsis and hemolysis in two patients with acute leukemia. J Pediatr Hematol Oncol. 1999; 21:431–435.

7. Gaur AH, Patrick CC, McCullers JA, Flynn PM, Pearson TA, Razzouk BI, et al. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin Infect Dis. 2001; 32:1456–1462.

8. Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. 2003; 41:3441–3444.
9. John AB, Razak EA, Razak EE, Al-Naqeeb N, Dhar R. Intractable Bacillus cereus bacteremia in a preterm neonate. J Trop Pediatr. 2007; 53:131–132.

10. Kiyomizu K, Yagi T, Yoshida H, Minami R, Tanimura A, Karasuno T, et al. Fulminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J Infect Chemother. 2008; 14:361–367.

11. Orrett FA. Fatal Bacillus cereus bacteremia in a patient with diabetes. J Natl Med Assoc. 2000; 92:206–208.
12. Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimura A, Hirai Y. Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis. 2011; 30:219–226.

13. Dohmae S, Okubo T, Higuchi W, Takano T, Isobe H, Baranovich T, et al. Bacillus cereus nosocomial infection from reused towels in Japan. J Hosp Infect. 2008; 69:361–367.

14. Barrie D, Hoffman PN, Wilson JA, Kramer JM. Contamination of hospital linen by Bacillus cereus. Epidemiol Infect. 1994; 113:297–306.
15. Helgason E, Tourasse NJ, Meisal R, Caugant DA, Kolsto AB. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl Environ Microbiol. 2004; 70:191–201.

16. Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998; 95:3140–3145.

17. Mignot T, Denis B, Couture-Tosi E, Kolsto AB, Mock M, Fouet A. Distribution of S-layers on the surface of Bacillus cereus strains: phylogenetic origin and ecological pressure. Environ Microbiol. 2001; 3:493–501.

18. Berger SA. Pseudobacteremia due to contaminated alcohol swabs. J Clin Microbiol. 1983; 18:974–975.

19. Weber DJ, Sickbert-Bennett E, Gergen MF, Rutala WA. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003; 289:1274–1277.

20. Maki DG. Through a glass darkly. Nosocomial pseudoepidemics and pseudobacteremias. Arch Intern Med. 1980; 140:26–28.

21. Ohsaki Y, Koyano S, Tachibana M, Shibukawa K, Kuroki M, Yoshida I, et al. Undetected Bacillus pseudo-outbreak after renovation work in a teaching hospital. J Infect. 2007; 54:617–622.

22. Morris T, Brecher SM, Fitzsimmons D, Durbin A, Arbeit RD, Maslow JN. A pseudoepidemic due to laboratory contamination deciphered by molecular analysis. Infect Control Hosp Epidemiol. 1995; 16:82–87.

23. Barker M, Thakker B, Priest FG. Multilocus sequence typing reveals that Bacillus cereus strains isolated from clinical infections have distinct phylogenetic origins. FEMS Microbiol Lett. 2005; 245:179–184.

24. Vassileva M, Torii K, Oshimoto M, Okamoto A, Agata N, Yamada K, et al. Phylogenetic analysis of Bacillus cereus isolates from severe systemic infections using multilocus sequence typing scheme. Microbiol Immunol. 2006; 50:743–749.

25. Raymond B, Wyres KL, Sheppard SK, Ellis RJ, Bonsall MB. Environmental factors determining the epidemiology and population genetic structure of the Bacillus cereus group in the field. PLoS Pathog. 2010; 6:e1000905.

26. Klee SR, Ozel M, Appel B, Boesch C, Ellerbrok H, Jacob D, et al. Characterization of Bacillus anthracis-like bacteria isolated from wild great apes from Cote d'Ivoire and Cameroon. J Bacteriol. 2006; 188:5333–5344.

27. Loeb M, Wilcox L, Thornley D, Gun-Munro J, Richardson H. Bacillus species pseudobacteremia following hospital construction. Can J Infect Control. 1995; 10:37–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download