Abstract
Purpose
This study aimed to examine the accuracy of rapid influenza diagnostic tests (RIDT) in children with an influenza-like illness and to evaluate factors associated with greater accuracy.
Methods
Pediatric patients, who visited Hallym University Sacred Heart Hospital with an influenza-like illness between June 2011 and May 2016, were enrolled in this study. We tested 798 samples using a real-time polymerase chain reaction (PCR) for respiratory viruses and compared the results with rapid influenza tests.
Results
In comparison with the results of the multiplex PCR, the positive agreement rates of RIDT for influenza A and B virus were 75.7% and 60.0%, respectively. The performance of RIDT varied according to days after fever onset. The positive agreement rates of RIDT for influenza A and B tests, performed within 4 days of fever onset, were 77.6% and 73.2%, but the rates for tests performed more than 5 days after fever onset were 66.7% and 21.4%, respectively.
Figures and Tables
Fig. 1
(A-E) Monthly distribution of children with influenza A & B infection detected by rapid influenza detection tests from June 2011 to May 2016 (sample total=1,774).

Table 1
Performance Characteristics of the Veritor and Sofia Methods Compared to the Polymerase Chain Reaction Method
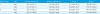
Values are presented as mean (95% confidence interval).
*Period of Veritor System (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) use: June 1, 2011 to December 31, 2014.
†Period of Sofia System (Quidel, San Diego, CA, USA) use: January 2, 2015 to May 31, 2016.
Abbreviations: RIDT, rapid influenza diagnostic test; PPV, positive predictive value; NPV, negative predictive value.
References
1. American Academy of Pediatrics. Pneumococcal infections. In : Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Committee on Infectious Diseases American Academy of Pediatrics. Red book: 2009 report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village: American Academy of Pediatrics;2009. p. 524–535.
2. Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006; 355:31–40.

3. Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000; 283:1016–1024.

4. Seo ES, Park GH, Kim SM, Kim SW, Jung WS, Cho KS, et al. Oseltamivir efficacy, side effects, and safety in children with influenza. Korean J Pediatr. 2010; 53:56–66.

5. Youn SE, Chun JH, Lee KS, Rha YH, Choi SH. Clinical characteristics of influenza B virus in children and the efficacy of oseltamivir: data from two university hospitals. Korean J Pediatr Infect Dis. 2014; 21:199–206.

6. Baas C, Barr IG, Fouchier RA, Kelso A, Hurt AC. A comparison of rapid point-of-care tests for the detection of avian influenza A(H7N9) virus, 2013. Euro Surveill. 2013; 18:20487.

7. Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012; 156:500–511.
8. Freymuth F, Vabret A, Cuvillon-Nimal D, Simon S, Dina J, Legrand L, et al. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006; 78:1498–1504.

9. Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006; 44:2382–2388.

10. Korea Centers for Disease Control and Prevention. Korean influenza surveillance report, 2012–2013 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;c2012. cited 2016 Dec 28. Available from: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0037-MNU1380&cid=21953.
11. Korea Centers for Disease Control and Prevention. Korean influenza surveillance report, 2013–2014 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;c2012. cited 2016 Dec 28. Available from: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0037-MNU1380&cid=60812.
12. Korea Centers for Disease Control and Prevention. Korean influenza surveillance report, 2014–2015 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;c2012. cited 2016 Dec 28. Available from: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0037-MNU1380&cid=66230.
13. Hwang Y, Kim K, Lee M. Evaluation of the efficacies of rapid antigen test, multiplex PCR, and real-time PCR for the detection of a novel influenza A (H1N1) virus. Korean J Lab Med. 2010; 30:147–152.

14. Gavin PJ, Thomson RB Jr. Review of rapid diagnostic tests for influenza. Clin Appl Immunol Rev. 2003; 4:151–172.

15. Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000; 160:3243–3247.

16. Kwon D, Shin K, Kwon M, Oh HB, Kang C, Lee JY. Development and evaluation of a rapid influenza diagnostic test for the pandemic (H1N1) 2009 influenza virus. J Clin Microbiol. 2011; 49:437–438.

17. Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011; 60:1–24.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download