Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Figure 1
Trend of probiotics usage and incidence of invasive infection of Lactobacillus spp. and Saccharomyces cerevisiae. (A) For 19 years from 1998 to 2016, 24 cases of invasive infection of Lactobacillus spp. and S. cerevisiae has occurred. (B) During recent 15 years, the incidence of invasive infection of Lactobacillus spp. and S. cerevisiae has increased significantly with increasing use of probiotics (R2=0.53 [Lactobacillus spp.], R2=0.39 [S. cerevisiae], R2=0.70 [total]; each P for trend <0.05). *Incidence of invasive infection is expressed as number of cases per year. †Probiotic consumption is expressed as days on the probiotics per 1,000 patient admission days per year.

Table 1
Demographics Characteristics of Patients with Invasive Infection caused by Lactobacillus spp. and Saccharomyces spp.

Values are presented as median (range) or number (%).
*Preterm is defined as babies born alive before 37 weeks of pregnancy are completed.
†Congenital heart diseases include functional single ventricle (n=2), double outlet ventricle with subaortic ventricular septal defect (n=1), complete atrioventricular septal defect (n=1), pulmonary atresia with ventricular septal defect (n=1), and tetralogy of Fallot (n=1).
‡Malignancy includes acute lymphoblastic leukemia (n=1), anaplastic large cell lymphoma (n=1), haemophagocytic lymphohistiocytosis (n=1), langerhans cell histiocytosis (n=1), and dysembryoblastic neuroepithelioma (n=1).
∫Neuromuscular disease includes Krabbe's disease (n=1), seizure disorder (n=1), and status epilepticus (n=1).
∥Chronic GI disorder includes necrotizing enterocolitis (n=1), autoimmune enteropathy (n=1), and meconium plug syndrome (n=1).
¶Chronic lung disease includes cystic fibrosis (n=1) and cytomegalovirus pneumonitis (n=1).
**Others includes brachial cleft cyst (n=3).
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; ANC, absolute neutrophil count.
Table 2
Clinical Manifestations and Outcomes of Invasive Infection caused by Lactobacillus spp. and Saccharomyces spp.

Values are presented as number (%) or median (range).
*Polymicrobial infection includes coinfection and superinfection.
†Coinfection includes 3 cases of bacteremia caused by Bacteroides caccae, Staphylococcus aureus, Neisseria sicca, respectively.
‡Superinfection includes 1 cases of fungemia caused by Candida glabrata .
∫CLABSI is defined10) as a laboratory-confirmed bloodstream infection where central line or umbilical catheter was in place for >2 calendar days on the date of event, with day of device placement being day 1, and the line was also in place on the date of event or the day before.
∥Deep seated infection includes infected bronchial cleft cyst (n=2), and wound infection (n=1).
¶An episode resulting in intensive care unit stay, mechanical ventilation, vasopressors, and inotropics and/or death was considered to be severe disease.
Abbreviations: CLABSI, central line-associated blood stream infection; ICU, intensive care unit.
Table 3
Description of Five Fatal Cases*
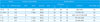
*There was no evidence of polymicrobial infection including superinfection and coinfection.
†Risk factor includes ICU stay before infection, presence of CL, and IC.
Abbreviations: ICU, intensive care unit; CL, central line; IC, immunocompromised state; FSV, functional single ventricle; L, Lactobacillus, spp.; MEM, meropenem; VAN, vancomycin; HLH, haemophagocytic lymphohistiocytosis; S, Saccharomyces cerevisiae; TZP, piperacillin-tazobactam; LAB, liposomal amphotericin B; LCH, langerhans cell histiocytosis; AMK, amikacin; CLR, clarithromycin; CST, colistin; AMB, amphotericin B deoxycholate; DORV, double outlet ventricle; IPM, imipenem; TOF, tetralogy of Fallot.
Table 4
Comparison of Demographic and Predisposing Factors for Clinically Severe Infections*

Values are presented as median (range) or number (%).
*An episode resulting in intensive care unit stay, mechanical ventilation and/or death was considered to be severe disease.
†Logistic regression analysis was carried out including the factors that were significantly different depending on clinical severity.
‡With a reference of Lactobacillus.
Abbreviations: OR, odds ratio; CI, confidence interval; NA, not applicable; GI, gastrointestinal; ICU, intensive care unit; ANC, absolute neutrophil count.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download