Abstract
Purpose
Severe combined immunodeficiency (SCID) is the most serious form of primary immunodeficiency. Infants with SCID are susceptible to life-threatening infections. To establish newborn screening for SCID in Korea, we performed a screening test for T—cell receptor excision circle (TREC) and K-deleting recombination excision circle (KREC) in neonates and investigated the awareness of SCID among their parents.
Methods
Collections of dried blood spots from neonates and parent surveys were performed at the Samsung Medical Center and Cheil General Hospital & Women's Healthcare Center in Korea. The amplification crossing point (Cp) value <37.0 was defined as TREC/KREC—positive based on cutoff values from measuring multiplex realtime polymerase chain reaction. A Cp value >39.0 was defined as negative.
Results
ForTREC/KREC screening, 141 neonates were enrolled; 63 (44.7%) were male. One hundred forty neonates (99.3%) had positive TREC/KREC results at the time of the initial test; 82.3% and 75.9% were positive and 17.0% and 23.4% were weakly positive for TREC and KREC, respectively. In one neonate (0.7%), the initial TREC/KREC test result was negative. However, repeated tests obtained and confirmed a positive result. For an awareness survey, 168 parents were engaged. Only 2% of parents (3/168) knew that the newborn screening test for SCID had been introduced and performed in other countries. Eighty—four percent of parents (141/168) replied that nationwide newborn SCID screening should be performed in Korean newborns.
REFERENCES
1. Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts IL, et al. Hematopoietic stem—cell transplantation for the treatment of severe combined immunodeficiency. N Engl I Med. 1999; 340:508–16.

2. Brown L, Xu—Bayford I, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011; 117:3243–6.

3. Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000—2009. N Engl I Med. 2014; 371:434–46.

4. Immune Deficiency Foundation (IDF). IDF SCID newborn screening campaign [Internet]. Towson: IDF;c2017. [cited 2017 Nov 7]. Available from:. https://primaryimmune. org.
5. GOV.UK. Population screening programmes [Internet]. GOV.UK.;. 2017. [cited 2017 Nov 7]. Available from:. https://www.gov.uk/topic/population—screening—programmes/newborn—blood—spot.
6. van Zelm MC, van der Burg M, Langerak AW, van Dongen II. PID comes full circle: applications of V(D) I recombination excision circles in research, diagnostics and newborn screening of primary immunodeficiency disorders. Front Immun01. 2011; 2:12.

7. Borte S, V0n Dobeln U, Fasth A, Wang N, Ianzi M, Wini-arski I, et al. Neonatal screening for severe primary immunodeficiency diseases using high—throughput triplex real—time PCR.B100d. 2012; 119:2552–5.
8. Kwan A, Abraham RS, Currier R, Brewer A, Andruszewski K, Abbott IK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. IAMA. 2014; 312:729–38.
9. Bousfiha A, Jeddane L, Al-Herz W, Ailal F, Casanova IL, Chatila T, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. I Clin Immunol. 2015; 35:727–38.

10. Chien YH, Chiang SC, Chang KL, Yu HH, Lee WI, Tsai LP, et al. Incidence of severe combined immunodeficiency through newborn screening in a Chinese population. J Formos Med Assoc. 2015; 114:12–6.

11. Rhim IVV, Kim KH, Kim DS, Kim BS, Kim IS, Kim CH, et al. Prevalence of primary immunodeficiency in Korea. I Korean Med 5C1. 2012; 27:788–93.

12. Centerwall WR, Chinnock RF, Pusavat A. Phenylketonuria: screening programs and testing methods. Am I Public Health Nations Health. 1960; 50:1667–77.

13. Puck IM. The case for newborn screening for severe combined immunodeficiency and related disorders. Ann N Y Acad 5C1. 2011. 12462108–17.

Fig. 1.
Schematic illustration for newborn TREC/KREC screening using multiplex realtime polymerase chain reaction (PCR). Abbrevaitions: TREC, T-cell receptor excision circle; KREC, K-deleting recombination excision circle; Cp, crossing point.
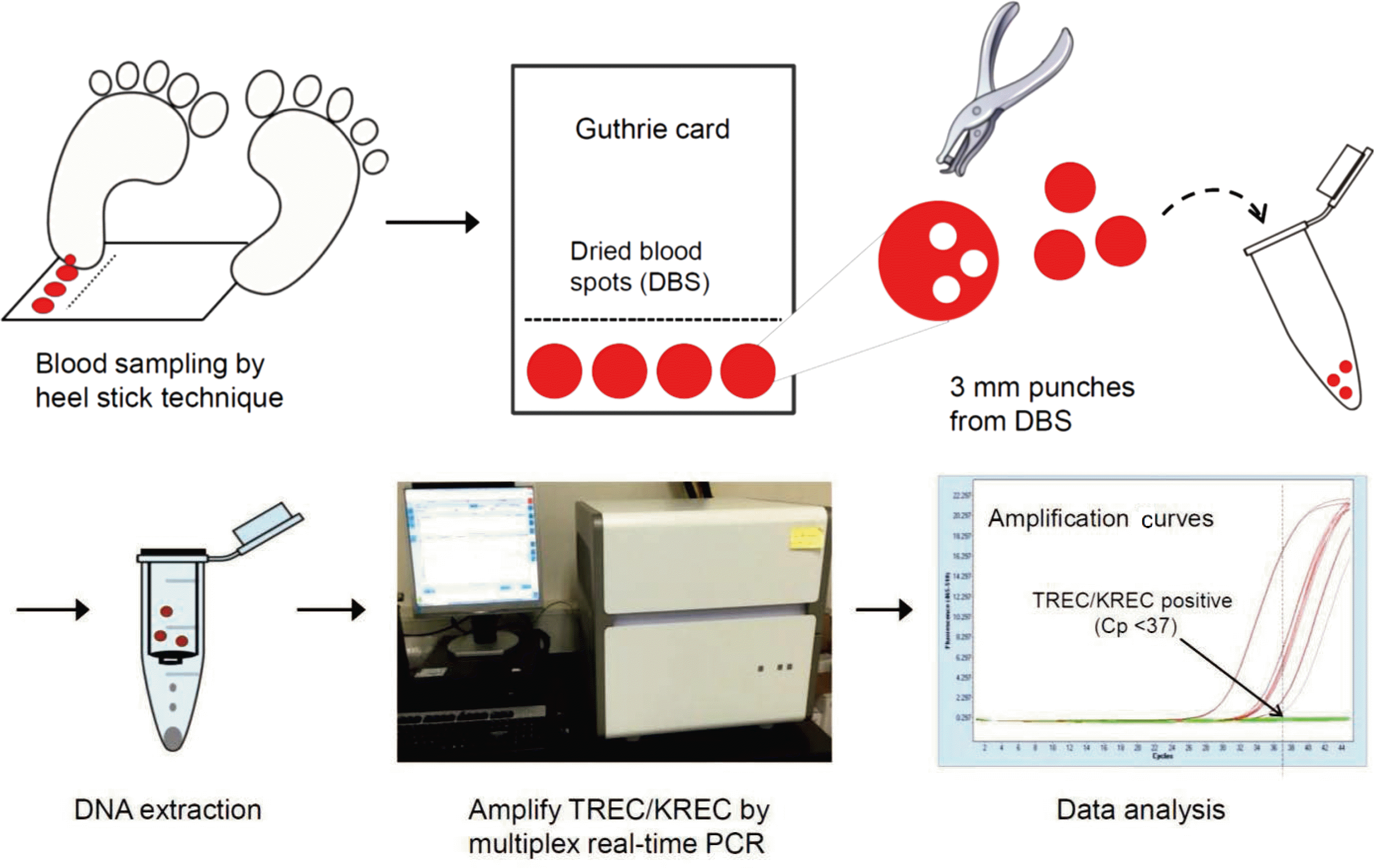
Fig. 2.
Flow chart of the multiplex realtime polymerase chain reaction for 141 freshly collected Guthrie cards from Korean newborns. Abbreviations: TREC, T-cell receptor excision circle; KREC, K-deleting recombination excision circle; Cp, crossing point.
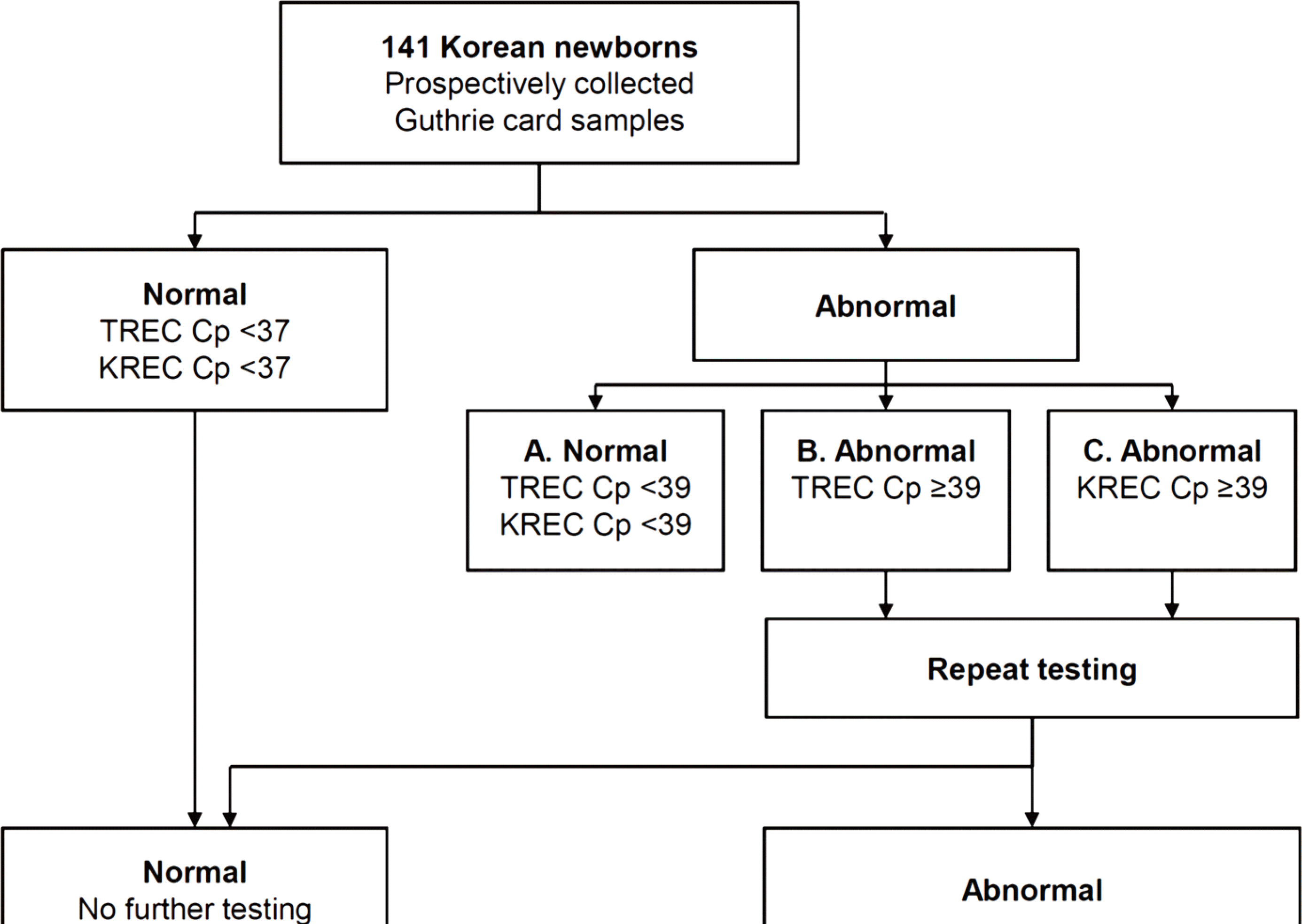
Fig. 3.
T-cell receptor excision circle (TREC) and K-deleting recombination excision circle (KREC) crossing point (Cp) values in dried blood spot samples from 141 neonates and negative controls.
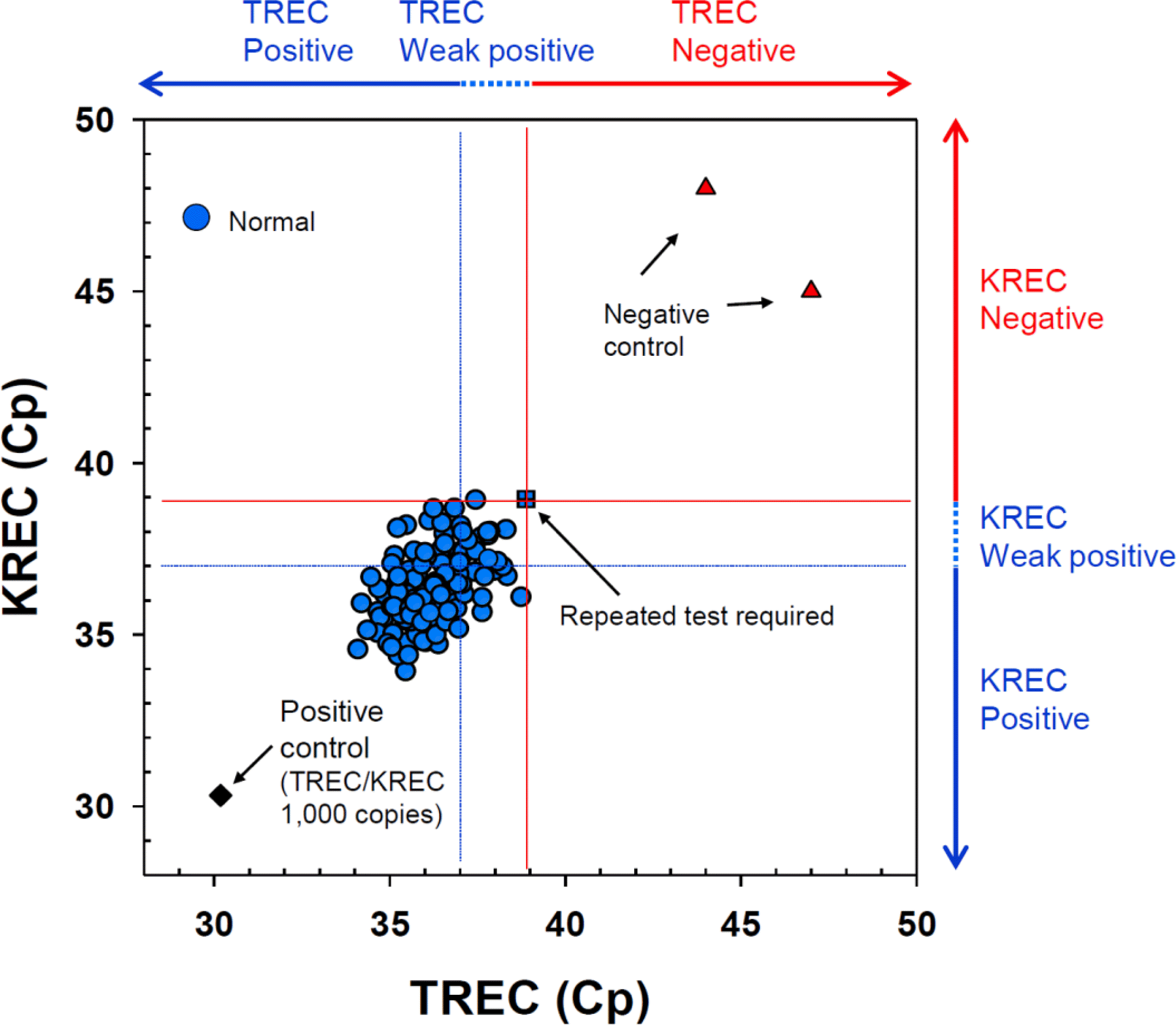
Fig. 4.
Questionnaire survey in expecting parents. A total of 168 parents answered. (A) Have you ever heard of newborn screening test for congenital metabolic diseases performed free of charge after birth in Korea? (B) Have you ever heard of primary immunodeficiencies? (C) Have you ever heard of severe combined immunodeficiency (SCID)? (D) Have you ever heard of newborn screening test for SCID that has been introduced and performed in other developed countries? (E) Do you think that newborn SCID screening is needed in Korean newborns?
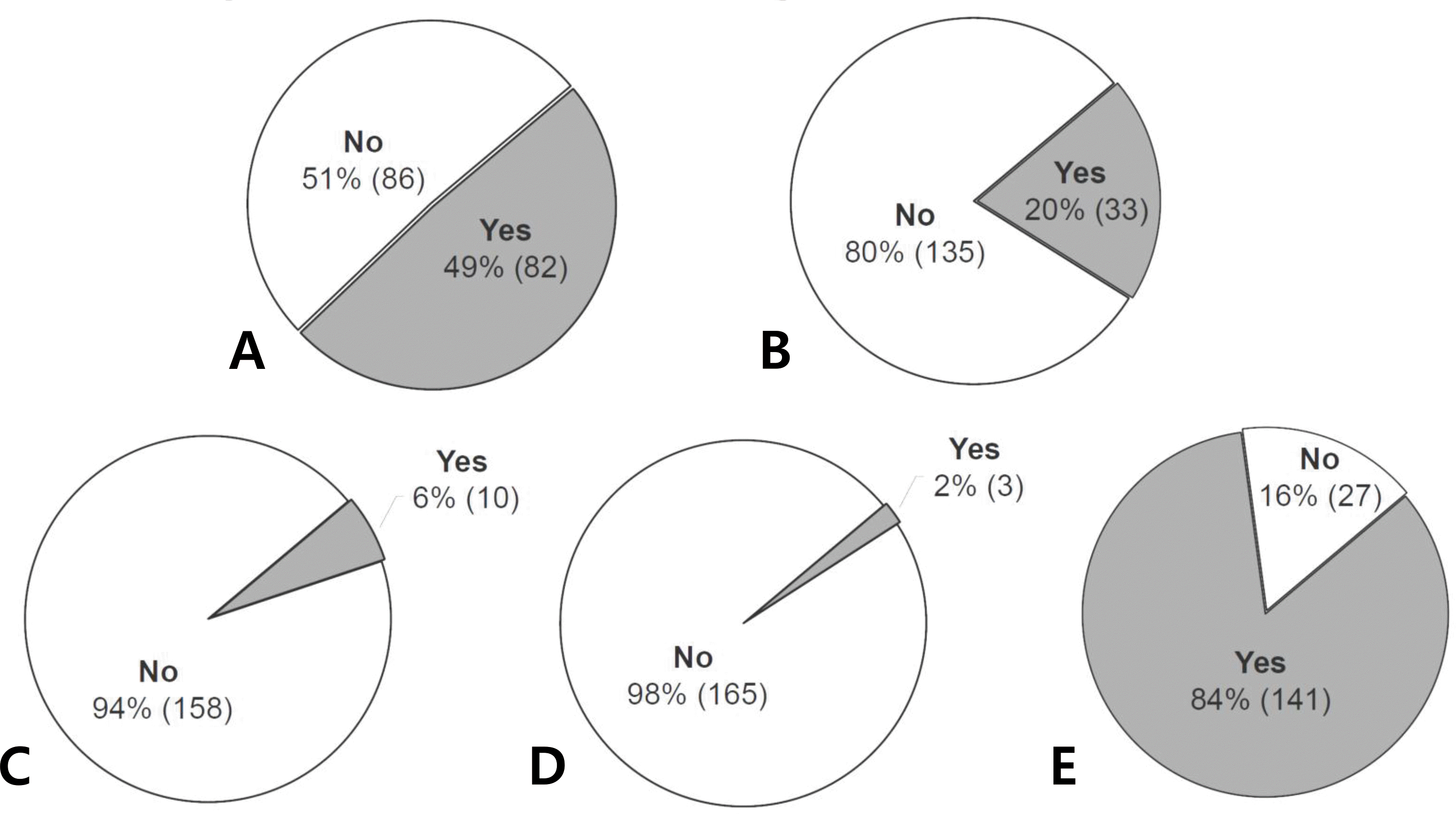
Table 1.
Analysis of TREC and KREC Cp Values
| Positive | Weak positive | Negative | |
|---|---|---|---|
| TREC | 83.0(117/141) | 17.0 (24/141) | 0(0/141)∗ |
| KREC | 76.6 (108/141) | 23.4 (33/141) | 0 (0/141)∗ |
Values are presented as percentage (number). ∗In one neonate, initial TREC/KREC test result was negative (Cp:39.9/40.0). How ever, repeated test confirmed positive (Cp=37.8/37.2, TREC/KREC weak positive). Abbreviations: TREC, T-cell receptor excision circle; KREC, K-deleting recombination excision circle Cp, crossing point.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download