Abstract
Blackwater fever is a serious clinical syndrome manifested by acute intravascular hemolysis, fever, and the passage of black or red urine, which is classically associated with falciparum malaria and irregular administration of quinine. In Korea, Plasmodium vivax is the only endemic malaria circulating; a number of imported cases of falciparum malaria have been reported in patients following return from international travel to a malaria endemic area. Therefore, it is important for health care professionals including pediatricians to be aware of the falciparum malaria and its clinical courses. Herein, we report a case of a 14-year-old girl with severe falciparum malaria that was complicated by blackwater fever.
Malaria is caused by intracellular Plasmodium protozoa transmitted to humans by female Anopheles mosquitoes1). The species known to infect humans are P. falciparum, P. vivax, P. ovale, P. malariae , and P. knowlesi . Among the species, P. falciparum causes severe clinical courses and is classically associated with significant morbidities and case fatality2). The World Health Organization defines a case of severe malaria as clinical or laboratory evidence of vital organ dysfunction, which include impaired consciousness, acidosis, hypoglycemia, severe malarial anemia, renal impairment, jaundice, pulmonary edema, significant bleeding, shock, and hyperparasitemia (>10%)3).
Blackwater fever is a serious clinical syndrome manifested by acute intravascular hemolysis, fever, and the passage of black or red urine. It is classically associated with recent or concurrent malaria and with the often irregular administration of quinine. It is not a common complication, but may cause renal failure and death in affected one4).
Korea is endemic to vivax malaria since its reemergence in 1993, and the cases are mostly reported from soldiers and adults residing in the region adjacent to demilitarized zone (DMZ)5). However, there are changes in epidemiology since then. Following the increased number of international travel among young adults and adolescents, more cases of imported malaria are noted recently6).
As the increase in the cases of imported malaria among Korean children is expected, review of its clinical manifestation and complications are of importance for clinicians. In this paper, we sought to report a rare case of blackwater fever complicated from imported severe falciparum malaria in a 14-year-old Korean girl.
On January 22, 2014, a previously healthy 14-year-old girl, who had returned 10 days earlier from a 12-day trip to Uganda presented with fever, vomiting, and diarrhea for 3 days. She did not take medications for malaria prophylaxis before the travel. She had a history of mosquito bites but denied fever during her stay in Uganda. When symptom was noticed at first, she visited a primary care clinic, and was treated under the impression of acute gastroenteritis. The patient had visited another clinic because she had shown new symptoms of nausea and dizziness. Her laboratory findings revealed thrombocytopenia and disseminated intravascular coagulation. The patient was then transferred to the emergency room of Seoul National University Hospital under the impression of septic shock.
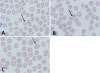
The initial vital signs were as follows: blood pressure, 79/48 mm Hg; pulse rate, 133 beats per minute; respiratory rate, 20 breaths per minute; and axillary temperature, 38.0℃. The patient was alert but acutely illlooking. Her sclerae were not icteric. The liver was palpable at two fingerbreadths and spleen was palpable at one fingerbreadth. Initial laboratory data included the following (Table 1): white blood cell, 9.45 ×10 3 /µL; hemoglobin, 13.3 g/dL; hematocrit, 37.1%; platelet, 21×10 3 /µL; sodium, 131 mmol/L; potassium, 3.6 mmol/L; chloride, 99 mmol/L; bicarbonate, 18 mmol/L; total calcium, 6.9 mg/dL; phosphorus, 1.4 mg/dL; blood urea nitrogen, 29 mg/dL; creatinine, 1.64 mg/dL; aspartate aminotransferase, 67 IU/L; alanine aminotransferase, 40 IU/L; albumin 3.0 g/dL; total bilirubin, 2.8 mg/dL; lactate dehydrogenase, 587 IU/L; C-reactive protein, 23.95 mg/dL (reference range, 0 to 0.5 mg/dL); and prothrombin time international normalized ratio (INR), 1.48 INR. Stained peripheral blood smears showed that 3% of the red blood cells were infected with P. falciparum (Fig. 1). The clinical diagnosis was severe malaria.
The patient was admitted to the pediatric intensive care unit for close monitoring and management. Oral artemether and lumefantrine was administered because quinine was not available. Clindamycin was administered intravenously, and was subsequently replaced by intravenous vancomycin and meropenem in order to cover possibly combined bacterial infection. Intravenous inotropic drugs were infused continuously. The next day, stained blood smears showed 10% of the red blood cells were infected with malarial parasite. Clinical condition has improved; therefore, the patient was transferred to the general ward. On the 3rd day, blood smears showed no parasitized red blood cells for the first time. On the 4th day, inotropic was tapered off. On the 7th day, even though the patient's blood smears were negative for 3 consecutive days, her parents insisted on the use of quinine. Antimalarial therapy was switched to quinine. On the 8th day, fever subsided, but however reappeared on the 9th day. On the 10th day, quinine and clindamycin were discontinued. On the 12th day, quinine was restarted because of sustained high fever. Red blood cell transfusion was done in response to the low hemoglobin level at 4.9 g/dL. The direct Coombs test was positive but indirect Coombs test was negative. The direct antiglobulin test was positive and haptoglobulin was <7 mg/dL, while plasma hemoglobin was elevated at 96.2 mg/dL. On the 13th day, the dark red-colored urine was noted and creatinine level has slightly increased to 1.39 mg/dL. Blood smears revealed negative for malaria. C-reactive protein was not elevated compared with the previous result and blood culture was negative (Table 1). Quinine was considered as a possible cause of current clinical manifestation, namely blackwater fever; however, was not discontinued after all because the parents insisted on the use of quinine to treat their child. On the 14th day, her fever was subsided and her hemoglobin level was elevated as 9.1 g/dL. On the 16th day, quinine treatment was completed for a total 7 days. On the 21st day, urine color has returned to yellow. On the 27th day, the patient was discharged with no sequela.
We report the first case of blackwater fever following severe falciparum malaria in a Korean child. The initial presentation of malaria is nonspecific, which may include fever, chills, headache, abdominal pain, diarrhea, or back pain78). These non-specific symptoms are also present in many other infections; therefore, the diagnosis of malaria can be missed easily. In Korea, where transmission of malaria is usually confined to the northern border, pediatricians should consider malaria as one of the differential diagnosis if the child has had a history of domestic travel near the DMZ area or international travel. Definitive diagnosis is obtained by microscopic demonstration of malaria parasites on stained blood smears. If initial blood smears are negative but the patient is still suspected of having malaria, the smear should be repeated every 12 to 24 hours for 72 hours9). The choice of antimalarial drug is based on the species, potential drug resistance, and the severity of disease7). The patient's travel history provides the most important clue for prescribing an appropriate antimalaria l drug9). Therefore, clinicians should always check an up-to-dated information about malaria endemicity and drug resistance where malaria patients traveled through readily available website provided by public health agencies (i.e., http://www.cdc.gov/malaria)7). In this case, the patient visited Uganda for 12 days. Uganda is estimated to be a high risk area of malaria for United States travelers by Centers for Disease Control and Prevention, and the incidence of malaria in Uganda was about 210 cases per 1,000 population in 2015. P. falciparum is the major species accounting for over 85%, and generally has chloroquine resistance.
The current recommendation states that patients with severe malaria require intensive care and immediate initiation of treatment of intravenous quinine combined with tetracycline, doxycycline, or clindamycin789). In one meta-analysis that systematically reviewed the seven trials with 929 participants, clindamycin plus quniine was found to be effective treatment option compared to artemisinin-based combination therapy recommended as standard care in the treatment of uncomplicated falciparum malaria10). This combination can be safely administered to both pregnant women and children. Recently, some have reported effectiveness of exchange transfusion in severe malaria cases with high parasitemia proportion (over 10%) in children and adults211).
Blackwater fever is a phenomenon demonstrated as acute intravascular hemolysis with hemoglobinuria, and a dramatic fall in hemoglobin value with scant or absent parasitemia. It usually occurs in patient living in malaria endemic area for long period, and is associated with irregular taking of amino-alcohol drugs (e.g., quinine, halofantrine, or mefloquine) for prophylaxis and treatment purpose12).
The pathogenesis of blackwater fever remains unclear; however, hypotheses had been postulated previously. Historically, the dramatic decrease in incidence of blackwater fever was evident when quinine was superseded by chloroquine in 195013) and the reemergence was shown after the reintroduction of quinine because of the development of resistance to chloroquine in P. falciparum14). This historical context strongly suggests that quinine plays a major role in the development of blackwater fever15). In a French study to investigate the potential causative agent of blackwater fever, quinine was the possible potential trigger in 29.5% of cases, halofantrine in 26.5% of cases, and mefloquine in 17.5% of cases. The median time interva l from the last administration of these drugs to the onset of blackwater fever was 24 hours (range, 5 to 120 hours)15).
In the patient presented in this report, there are clinical evidences to suspect the blackwater fever: a recent stay in a malaria endemic area, confirmed infection of P. falciparum, intermittent administration of multiple antimalarial drugs including quinine, recurrent fever, abrupt hemolysis, and hemoglobinuria in the absence of parasitemia. There is no evidence of other infections or cause of hemolysis in laboratory examination. Quinine and lumefantrine are potential causative agents of blackwater fever in this case on the previous report16).
When the blackwater fever is suspected, suspected causative drugs should be withdrawn immediately. Meanwhile, recent study based on blackwater fever in 1996 demonstrated the case fatality rate of 2% even though antimalarial treatment had not been discontinued4). Supportive medical care is a critical part in the treatment of blackwater fever. In this case, although we had to continue quinine because of parents' choice, the patient recovered completely following medical supportive care including transfusion and balanced fluid management. Because treatment of blackwater fever is different from that of malaria with persistent parasitemia, it is important to establish a definite diagnosis. Moreover, switch to the antimalarial drugs such as sulfadoxine-pyrimethamine, artemisinin derivatives, and atovaquone-proquanil which do not have crossreactivity with presumed triggering drug could be another option15).
In Korea, P. vivax is endemic and causes less than 1,000 cases annually since 201217). In contrast, imported malaria was chiefly caused by P. falciparum which the most severe clinical manifestation among the five species. As the international travel from Korea to malarious areas tended to increase, the incidence of imported malaria has increased from 30 persons/year before 2010 to 71 persons/year in 201518). As the risk of falciparum malaria is expected to continue, physicians should advocate pretravel counselling for appropriate prophylaxis and be prepared for its treatment. Our patient did not have pretravel counselling prior to travel to Uganda; therefore, had missed opportunity to prevent such infection. This case gives us lesson to note such infection in children with recent travel history and to be aware of possible complications during the course of treatment.
Blackwater fever should be distinguished from persistent malaria because their managements are different . As in this case, if red or black urine and recurrent fever without parasitemia is evident during the course of antimalarial treatment, appropriate management plan should be established in consideration of blackwater fever, which is a rare but one of potential complication s following antimalarial treatment.
Figures and Tables
Fig. 1
Different morphological characteristics of Plasmodium falciparum observed in the case patient: (A) marginal form; (B) binucleate form; (C) polyparasitism (thin film; Wright-Giemsa stain, ×1,000).
References
1. John CC. Malaria (Plasmodium). Kliegman RM, Stanton BM, St Geme J, Schor NF, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Elsevier;2015. p. 1709–1721.
2. Boctor FN. Red blood cell exchange transfusion as an adjunct treatment for severe pediatric falciparum malaria, using automated or manual procedures. Pediatrics. 2005; 116:e592–e595.

3. World Health Organization. Severe malaria. Trop Med Int Health. 2014; 19:Suppl 1. 7–131.
4. Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996; 23:1274–1281.

5. Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, et al. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003; 69:159–167.

6. Korea Centers for Disease Control and Prevention. Epidemiologic characteristics of imported malaria in Korea, 2003-2012 [Internet]. Cheongju: KCDC;c2012. cited 2017 May 30. Available from: http://cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0037-MNU1380&cid=22134.
7. American Academy of Pediatrics. Malaria. Kimberlin DW, Long SS, Brady MT, Jackson MA, editors. Red book: 2015 report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village: American Academy of Pediatrics;2015. p. 528–535.
8. Lee SY, Ko TS, Chi HS, Park YS. Four cases of the imported falciparum malaria in children. J Korean Pediatr Soc. 1997; 40:249–254.
9. Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA. 2007; 297:2264–2277.
10. Obonyo CO, Juma EA. Clindamycin plus quinine for treating uncomplicated falciparum malaria: a systematic review and meta-analysis. Malar J. 2012; 11:2.

11. Chung HS, Peck KR, Kim DW. Two case reports of successful therapeutic erythrocytapheresis as an adjunctive therapy in severe falciparum malaria. Ther Apher Dial. 2010; 14:230–233.

12. World Health Organization, Division of. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990; 84:Suppl 2. 1–65.

13. Bruce-Chwatt LJ. Quinine and the mystery of blackwater fever. Acta Leiden. 1987; 55:181–196.
14. Delacollette C, Taelman H, Wery M. An etiologic study of hemoglobinuria and blackwater fever in the Kivu Mountains, Zaire. Ann Soc Belg Med Trop. 1995; 75:51–63.
15. Bruneel F, Gachot B, Wolff M, Regnier B, Danis M, Vachon F, et al. Resurgence of blackwater fever in long-term European expatriates in Africa: report of 21 cases and review. Clin Infect Dis. 2001; 32:1133–1140.

16. de Villiers KA, Marques HM, Egan TJ. The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J Inorg Biochem. 2008; 102:1660–1667.

17. World Health Organization. World malaria report 2015 [Internet]. Geneva: World Health Organization;c2017. cited 2017 May 30. Available from: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/.
18. Korea Centers for Disease Control and Prevention. Guidelines for malaria prevention and control 2016 [Internet]. Cheongju: KCDC;c2012. cited 2017 May 30. Available from: http://cdc.go.kr.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download