Abstract
Purpose
Coronary arterial lesions (CALs) were reported to have developed in children with systemic inflammatory diseases, as well as those with Kawasaki disease (KD). The purpose of this study was to confirm that the CAL development in children with KD occurs in a mouse model of sepsis presenting typical systemic inflammatory response syndrome (SIRS).
Methods
To induce the sepsis mouse model with SIRS, 6-week-old C57BL/6 mice were intraperitoneally injected with endotoxin. We compared histological findings of the major organs between the control and the sepsis groups and examined CAL in the heart of the septic mice.
Results
Infiltrating inflammatory cells were relatively increased in the heart, liver, and kidneys of the sepsis group, compared with those of the control group. We confirmed lymphocytic infiltration in the myocardium (myocarditis) and the pericardial soft tissue of the heart. Furthermore, coronary artery of the septic mouse was identified, but CAL was not observed.
Conclusions
In this study, we failed to confirm the existence of CAL in a mouse model of sepsis. However, it is well-known that CALs are seen in many kinds of diseases that cause SIRS. Our findings suggest further investigation into the clinical significance of CAL in various systemic inflammatory diseases, including KD.
Figures and Tables
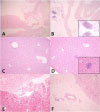 | Fig. 1Histologic findings (H&E stain) of the major organs are compared between the control and the sepsis groups. (A) Heart, control: unremarkable (×100). (B) Heart, sepsis: lymphocytic infiltration in the myocardium and the pericardial soft tissue (×40; inset, ×200). (C) Liver, control: unremarkable (×100). (D) Liver, sepsis: a few microabscesses (×100; inset, ×200). (E) Kidney, control: unremarkable (×100). (F) Kidney, sepsis: lymphocytic infiltration in the perirenal soft tissue (×100). |
References
1. Kaplan SL, Vallejo JG. Bacteremia and septic shock. In : Cherry JD, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez P, editors. Feigin and Cherry's textbook of pediatric infectious diseases. 7th ed. Philadelphia: Saunders/Elsevier;2014. p. 824–836.
2. Lee SY, Lee KH, Hwang HS, Jeong DC, Chung SY, Kang JH. Septic encephalopathy complicating acute appendicitis. Pediatr Crit Care Med. 2009; 10:e11–e13.

3. Zanotti-Cavazzoni SL, Goldfarb RD. Animal models of sepsis. Crit Care Clin. 2009; 25:703–719.
4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–2771.

5. Yun HW, Lee JY, Yang SI, Yu HJ, Kang MJ, Lee SY, et al. Comparison of cervical-lymph-node-first presentation of Kawasaki disease and typical Kawasaki disease. Pediatr Infect Vaccine. 2016; 23:10–17.

6. Park HJ, Cho YJ, Bae EY, Choi UY, Lee SY, Jeong DC, et al. Macrophage activation syndrome as the extreme form of Kawasaki disease. Korean J Pediatr Infect Dis. 2010; 17:177–181.

7. Lee SY, Han JW. Animal model of Kawasaki disease. Pediatr Clin Immunol. 2012; 4:46–49.
8. Rached-D'Astous S, Boukas I, Fournier A, Raboisson MJ, Dahdah N. Coronary artery dilatation in viral myocarditis mimics coronary artery findings in Kawasaki disease. Pediatr Cardiol. 2016; 37:1148–1152.
9. Binstadt BA, Levine JC, Nigrovic PA, Gauvreau K, Dedeoglu F, Fuhlbrigge RC, et al. Coronary artery dilation among patients presenting with systemic-onset juvenile idiopathic arthritis. Pediatrics. 2005; 116:e89–e93.

10. Agarwal A, Biglarian S, Lim-Stavros S, Votava-Smith JK, Ramanathan A. Pediatric systemic lupus erythematosus presenting with coronary arteritis: a case series and review of the literature. Semin Arthritis Rheum. 2015; 45:42–47.

11. Gatterre P, Oualha M, Dupic L, Iserin F, Bodemer C, Lesage F, et al. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. 2012; 38:872–878.

12. Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince JM, Levy RM, et al. In silico models of acute inflammation in animals. Shock. 2006; 26:235–244.

13. von Drygalski A, Furlan-Freguia C, Ruf W, Griffin JH, Mosnier LO. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arterioscler Thromb Vasc Biol. 2013; 33:769–776.

14. Wang D, Chen Y, Jiang J, Zhou A, Pan L, Chen Q, et al. Carvedilol has stronger anti-inflammation and anti-virus effects than metoprolol in murine model with coxsackievirus B3-induced viral myocarditis. Gene. 2014; 547:195–201.

15. Takahashi K, Oharaseki T, Wakayama M, Yokouchi Y, Naoe S, Murata H. Histopathological features of murine systemic vasculitis caused by Candida albicans extract: an animal model of Kawasaki disease. Inflamm Res. 2004; 53:72–77.

17. Kato S, Yoshimura K, Tanabe Y, Kimata T, Noda Y, Kawasaki H, et al. A child with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis complicated by coronary artery lesion mimicking Kawasaki disease. J Pediatr Hematol Oncol. 2013; 35:e317–e319.

18. Usta Guc B, Cengiz N, Yildirim SV, Uslu Y. Cytomegalovirus infection in a patient with atypical Kawasaki disease. Rheumatol Int. 2008; 28:387–389.

19. Boukas I, Dahdah N, Robitaille Y, Fournier A. Coronary artery dilatation and vasculitis in a case of rabies: similarity with Kawasaki disease. Pediatr Int. 2013; 55:237–240.

20. Hasan A, McDonough KH. The effects of Escherichia coli sepsis and short-term ischemia on coronary vascular reactivity and myocardial function. Shock. 1997; 8:305–310.

21. Wiesenthal AM, Todd JK. Toxic shock syndrome in children aged 10 years or less. Pediatrics. 1984; 74:112–117.

22. Yim D, Ramsay J, Kothari D, Burgner D. Coronary artery dilatation in toxic shock-like syndrome: the Kawasaki disease shock syndrome. Pediatr Cardiol. 2010; 31:1232–1235.

23. Gunal N, Baysal K, Haciomeroglu P, Belet N, Kolbakir F. Rheumatic heart disease and coronary vasculitis in children. Acta Paediatr. 2006; 95:118–120.

24. Ouali S, Kacem S, Ben Fradj F, Gribaa R, Naffeti E, Remedi F, et al. Takayasu arteritis with coronary aneurysms causing acute myocardial infarction in a young man. Tex Heart Inst J. 2011; 38:183–186.
25. Gurkan U, Kaya A, Tatlisu MA, Avsar S. A case report of coronary artery aneurysm in a patient with Behcet's disease. Turk Kardiyol Dern Ars. 2014; 42:651–654.

26. Christou A, Patsilinakos S, Chinofoti I, Marinakos A, Nikolaou N, Spanodimos S. Acute myocardial infarction in a patient with coronary artery aneurysm and Crohn's disease. Hellenic J Cardiol. 2012; 53:400–402.
27. Perez-Colon E, Dadlani GH, Wilmot I, Miller M. Mesalamineinduced myocarditis and coronary vasculitis in a pediatric ulcerative colitis patient: a case report. Case Rep Pediatr. 2011; 2011:524364.

28. Serrano R, Martinez MA, Andres A, Morales JM, Samartin R. Familial mediterranean fever and acute myocardial infarction secondary to coronary vasculitis. Histopathology. 1998; 33:163–167.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download