Abstract
Nail unit melanoma is a type of acral lentiginous melanoma and requires histopathologic examination for a confirmed diagnosis. However, inadequate biopsy techniques make definitive diagnosis difficult. A 61-year-old man presented with progressive nail pigmentation for 15 years, which was clinically highly suspicious for malignancy. Acral lentiginous melanoma was not detected in punch and longitudinal biopsy specimens, but en bloc excision tissue revealed melanoma. Acral lentiginous melanoma is known to have a heterogeneous pathologic manifestation depending on the pigmented region and the time it takes to progress. In this regard, en bloc excision can be considered as a first-line biopsy technique to diagnose acral lentiginous melanoma, indolent subtype.
Subungual melanoma, which commonly presents as linear melanonychia, is a rare type of cutaneous melanoma. It accounts for about 2% of all melanoma in the white population, and 10%~18% of that in the Asian population123. It usually originates from the nail matrix, and then extent to the other sites of nail unit: nail bed, hyponychial, and nailfolds, resulting in pigmentation4. Many biopsy methods are described to obtain adequate tissue from the lesion: punch biopsy, longitudinal incision, and en bloc excision5. Herein, we report a case of invasive subungual melanoma, which was not diagnosed by punch and longitudinal incisional biopsy, but was diagnosed only by en bloc excision.
A 61-year-old man presented with left thumbnail pigmentation, which persisted for 15 years. It was accompanied by pain, tenderness, and a yellowish discharge. About 1-cm wide melanonychia, onychodystrophy in the middle portion, and Hutchinson's sign were observed in the nail (Fig. 1A). The pigmentation had initially appeared as a dot and progressed to linear melanonychia; then, it widened after a few years. Histopathologic examination by punch matrix biopsy conducted one year ago, in another dermatology clinic, revealed the pigmented lesion to be benign and that it caused onychodystrophy. Subsequently, a diagnostic incisional biopsy was performed, encompassing the lateral, proximal nail folds together with the nail matrix, and the hyponychium (Fig. 1B). Atypical melanocytes were not observed, and extensive pigmentation was prominent in the nail plate (Fig. 2A). Also only bland pigmentation without atypical melanocyte proliferation was observed in the nail bed and the hyponychium (Fig. 2B, C). Immunohistochemistry (IHC) revealed the following: S-100, positive (melanocytes); cyclin D1 and Ki-67, rarely positive; human melanoma black 45 (HMB45), negative. The lesion was reported to be benign. However, we referred the patient to a plastic surgeon for complete excision of the lesion, because the lesion was clinically suspicious and we could not totally exclude the possibility of malignant melanoma (Fig. 3A, B). Subsequently, in the en bloc excisional histopathologic specimen, atypical melanocytes with hyperchromatic nuclei were detected, which stained HMB45, cyclin D1, and Ki-67 positive in immunohistochemical examination (Fig. 3C~F). Atypical melanocytes invaded up to 0.4 mm of the dermal layer of the hyponychium. Based on this result, we established the diagnosis of acral lentiginous melanoma (ALM). Consequently, the patient underwent an amputation of the distal phalanx of his left thumb in the department of plastic surgery.
Levit et al.6 suggested the “ABCDEF” rule in diagnosing nail unit melanoma: age (5th to 7th decades), ethnicity (African Americans, Asians, and native Americans), brown to black colored bands of width more than 3 mm, any changes in the nail band, digit, and extension of pigment-Hutchinson's sign. These could be the signs of malignant melanoma, which would require a biopsy. However, the histopathological changes of early nail unit melanoma are often equivocal, which can result in a misdiagnosis. Discrepancy between the clinical and histopathological findings is frequently observed when a specimen is incompletely excised7. Likewise, all of the above ABCDEF criteria were positive in this case, clinically indicating malignant subungual melanoma. However, the initial histopathologic work-up, including the punch and longitudinal incisional biopsies failed to reveal the ALM focus. The possibility of malignancy in the surrounding pigmented region compelled us to perform an en bloc excision of the lesion, which finally confirmed invasive melanoma. Therefore, when clinical and histopathological findings do not concur, a further biopsy should be considered. Additionally, our results suggest that a part of the nail unit is not sufficient to establish a diagnosis because it cannot represent the whole lesion. The range of pigmentation does not verify the presence of malignant cells.
Nail unit melanoma belongs to the ALM subgroup. Recently, an indolent ALM subtype with a long radial growth phase was described8. The characteristics of this subtype include clinical malignant melanoma with scattered proliferation of atypical melanocytes in a long radial growth phase of many years and absence of dermal invasion. Although, atypia of melanocytes was not sufficient for the diagnosis of ALM in situ, the authors speculated that it was a very early stage of ALM in situ, because some of lesions appeared to have progressed to an invasive stage after a long time. Accordingly, we diagnosed our case as ALM, indolent subtype with a long radial growth phase. In these slowly progressing ALM lesions, histopathologic findings are sometimes misleading, especially when incompletely biopsied, as they show only a still image of the entire biologic process7.
Histopathologic and clinical analysis of the progression pattern of subungual melanoma showed that atypical melanocytes usually arise from the nail matrix and spread in several directions: horizontal extension causes the triangular sign, and distal extension occurs along the nail bed to the hyponychium (Hutchinson's sign) or the nail plate4910. This means that the existence of atypical melanocytes and the extent of their invasion could be variable in spots of the nail unit, both lengthwise and breadthwise. In this regard, Izumi et al.11 proposed a pathological characteristic that the tumor cells are more noticeable in the hyponychium than in the nail bed or matrix. In addition, Shin et al.1213 revealed that the nail matrix is more resistant to invasion, due to the presence of the onychodermis and the upward growth of the nail matrix. Based on these investigations, a longitudinal incisional biopsy that is inclusive all parts of the nail unit should be preferred over punch matrix biopsy, in order to avoid underdiagnosis1113. In this case, the incisional biopsy specimen does not appear to include the most severe area, which might have resulted in the lack of evidence of malignancy on histopathological examination. However, incisional biopsy from grossly severe area might not sufficient for a correct diagnosis of indolent ALM either, because even en bloc specimen revealed only small foci of invasion in this case.
There were several limitations in this case report. First, ALM could have been diagnosed if the longitudinal incisional biopsy specimen had been obtained in the median portion of the nail. The lateral portion was less pigmented than the median (Fig. 1A). Second, an additional IHC staining, like melan-A, could be helpful in finding unidentified melanocyte proliferation in the epidermis as melan-A is more sensitive than HMB45 in detecting melanocytic lesion14. Third, conservative surgical managements should have been considered rather than amputation in the treatment aspect. Recent study15 suggested that conservative surgical treatment is a procedure with good cosmetic and functional outcome along with compatible prognosis, in patients with in situ or minimally invasive subungual melanoma.
Herein, we present a case of ALM, indolent subtype with a long radial growth phase, which was diagnosed by en bloc excision. Our case is noteworthy because, although the clinical findings were strongly suggestive of ALM, histopathology of the specimens obtained from a punch biopsy and a longitudinal incisional biopsy showed subtle changes insufficient for the diagnosis of even very early ALM in situ. We suggest that the en bloc excision could be considered as a further investigating method to diagnose ALM, indolent subtype when other biopsy techniques fail to confirm the ALM in a highly suspicious nail.
Figures and Tables
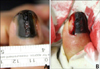 | Fig. 1(A) Dark black colored pigmented band with about 1-cm width on the left thumbnail. Ulceration, onychodystrophy, and Hutchinson's sign were observed. (B) Left lateral portion of the nail unit was longitudinally biopsied, enclosing nail folds, hyponychium, and nail matrix. |
 | Fig. 2(A) Prominent pigmentation in the nail plate without atypical melanocytes (H&E; ×100, inset: ×50). (B) Bland pigmentation was in the basal layer of hyponycium (H&E; ×100, inset: ×10). (C) Significant pigmentation was observed in the nail bed without atypical melanocytes (H&E; ×400, inset: ×100). |
 | Fig. 3(A) Two months later, the nail unit presented with plate destruction and intermittent bleeding from the nailbed. (B) En bloc excisional specimen of the nail unit. (C) Scattered pigmentation was observed in the hyponychium and the nail bed with lymphocytic infiltration in the onychodermis layer (H&E, ×10). (D) Atypical melanocytes with hyperchromatic nuclei invaded the dermis layer (H&E, ×100). (E, F) Immunohistochemical stains for human melanoma black 45 and Ki-67 were positive (×100). |
References
1. Dawber RP, Colver GB. The spectrum of malignant melanoma of the nail apparatus. Semin Dermatol. 1991; 10:82–87.
2. Ishihara K, Saida T, Yamamoto A. Japanese Skin Cancer Society Prognosis and Statistical Investigation Committee. Updated statistical data for malignant melanoma in Japan. Int J Clin Oncol. 2001; 6:109–116.

3. Kim JH, Park JH, Lee DY. Site distribution of cutaneous melanoma in South Korea: a retrospective study at a single tertiary institution. Int J Dermatol. 2015; 54:e38–e39.

4. Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, Quinn MJ, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007; 31:1902–1912.
5. Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol. 1989; 21:1165–1175.

6. Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000; 42:269–274.
7. Park HS, Cho KH. Acral lentiginous melanoma in situ: a diagnostic and management challenge. Cancers (Basel). 2010; 2:642–652.

8. Kim JY, Choi M, Jo SJ, Min HS, Cho KH. Acral lentiginous melanoma: indolent subtype with long radial growth phase. Am J Dermatopathol. 2014; 36:142–147.
9. Lee DY. Variable sized cellular remnants in the nail plate of longitudinal melanonychia: evidence of subungual melanoma. Ann Dermatol. 2015; 27:328–329.

10. Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol. 2010; 162:765–771.

11. Izumi M, Ohara K, Hoashi T, Nakayama H, Chiu CS, Nagai T, et al. Subungual melanoma: histological examination of 50 cases from early stage to bone invasion. J Dermatol. 2008; 35:695–703.

12. Shin HT, Park SW, Lee DY, Jang KT, Mun GH, Cheung L. A case of subungual melanoma with tumor invasion sparing the nail matrix dermis. Ann Dermatol. 2014; 26:655–657.

13. Shin HT, Jang KT, Mun GH, Lee DY, Lee JB. Histopathological analysis of the progression pattern of subungual melanoma: late tendency of dermal invasion in the nail matrix area. Mod Pathol. 2014; 27:1461–1467.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download