Abstract
Background
A new shampoo with anti-Malassezia properties obtained from various plants is required to provide seborrheic dermatitis patients with a wider range of treatment options.
Objective
The aim of this study was to obtain in vitro susceptibility profiles of Malassezia restricta and M. globosa, the most important pathogenic organisms in the development of seborrheic dermatitis, to the plant extracts used in commercial anti-dandruff shampoos.
Methods
Minimal inhibitory concentrations (MICs) were determined for eight candidate plant extracts and two plant-derived natural products diluted with Leeming and Notman medium to final concentrations of 0.016 to 1 mg/ml.
Results
Castanea crenata shell, Camellia sinensis leaf, and oil-soluble Glycyrrhiza extracts presented relatively low MIC values (≤0.5 mg/ml) against both strains. The C. crenata shell and oil-soluble Glycyrrhiza extracts demonstrated especially high anti-Malassezia activity, suggesting their potential use in the treatment of seborrheic dermatitis. The extracts also showed fungistatic activity against other common facultative pathogenic yeasts, Cryptococcus and Candida.
Seborrheic dermatitis (SD), including its minor form, dandruff, is an important and common abnormal skin condition affecting about 5% to 10% of the population12. It is characterized by flaking and scaling of the scalp, accompanied by itch and irritancy. The pathogenesis of SD is not completely understood yet, but a strong association with skin colonization by Malassezia yeasts has been suggested3.
Yeasts of the genus Malassezia are members of the human skin flora and are found in 75% to 98% of healthy adults4. Most Malassezia yeasts are lipid-dependent, requiring an external source of lipids. For this reason, these cutaneous fungi prevail in body areas rich in sebaceous glands, such as the scalp, face, and upper trunk5. However, with some predisposing factors, these yeasts can be etiological agents of several skin disorders and uncommon systemic infections. They have been considered opportunistic pathogens of SD, and other conditions, including pityriasis versicolor, atopic dermatitis, folliculitis, and seborrheic blepharitis6.
With the development of molecular methods, such as polymerase chain reaction, Malassezia taxonomy has undergone a great transformation, with the expansion of the genus to the 14 species known today. Among the 14 species, Malassezia restricta and M. globosa are recognized as the most important pathogenic organisms in the development of dandruff and SD. However, an association of M. sympodialis, M. slooffiae, Malassezia obtusa, and M. furfur with SD has also been reported in some studies4.
Owing to its chronic course with remissions and relapses, SD management presents a challenge to clinicians. The condition requires repeated treatment and regular prophylaxis. Medicated and some commercial shampoos containing synthetic antifungal agents in various formulations are frequently prescribed to patients with scalp SD. In particular, ketoconazole and zinc pyrithione shampoos are commonly prescribed78, but do not always have a good outcome. Recently, new shampoos containing natural ingredients, especially various plant extracts with anti-Malassezia activity, have been expected to provide patients with a wider range of treatment options.
A number of natural ingredients, especially extracts from various plants with known anti-inflammatory or antimicrobial activities, have been included in commercial shampoos for SD and dandruff. Some ingredients show better than expected effects on SD patients and are presumed to actually have anti-Malassezia activity. However, the activities of these natural ingredients against Malassezia infections have not been reliably identified yet based on appropriate laboratory determinations through in vitro susceptibility testing. In the present study, we evaluated the in vitro susceptibility of M. restricta and M. globosa, the major Malassezia species associated with SD, to eight plant extracts, as well as dipotassium glycyrrhizate and phytosphingosine, used in commercial and medicated dandruff shampoos.
The following eight candidate extracts and two plant-derived natural products were investigated: 3% Castanea crenata shell extract, 0.1% Camellia sinensis leaf extract, 10% Artemisia vulgaris extract, 1% oil-soluble Glycyrrhiza extract, 51% Astragalus membranaceus root extract, 64.1% Pinus sylvestris leaf extract, 75% Prunus mume fruit extract, 93.5% Coix lacryma-jobi var. ma-yuen seed extract, 100% dipotassium glycyrrhizate, and 100% phytosphingosine. All these are ingredients were added to anti-dandruff shampoos based on their anti-inflammatory effects and antimicrobial activities. They were provided by Amorepacific Corporation (Seoul, Korea) as manufactured products.
Two isolates, M. restricta CBS 7877 and M. globosa CBS 7966, previously deposited in a culture collection were used in initial experiments. These yeasts were isolated from clinical samples obtained from human patients with SD. The strains were grown in Leeming and Notman (LN) medium9. In addition, M. sympodialis KCTC 27514 and M. slooffiae KCTC 27517 were used to confirm the antifungal activity of the C. crenata shell and oil-soluble Glycyrrhiza extracts.
For further evaluation of the antifungal activity of the C. crenata shell and oil-soluble Glycyrrhiza extracts, we conducted additional experiments with Cryptococcus neoformans H99, Candida albicans SC5314, C. tropicalis KCTC 7512, and C. parapsilosis KCTC 7514.
Minimal inhibitory concentrations (MICs) were determined using the method suggested by Sugita et al.10 with some modifications. Briefly, the extracts/reagents were diluted with 980 µl melted LN agar (LNA) medium to final concentrations ranging from 0.016 to 1 mg/ml. Malassezia species were grown in each well and incubated for three days at 34℃ to determine MICs.
We further compared the antifungal activity of the C. crenata shell and oil-soluble Glycyrrhiza extracts to that of ketoconazole. C. crenata shell extract, oil-soluble Glycyrrhiza extract, and ketoconazole were diluted with 980 µl melted LNA medium to final concentrations ranging from 15.63 to 1,000 µg/ml and 0.015 to 7.68 µg/ml, respectively. Malassezia strains were grown in each well of a 24-well plate and incubated for three days at 34℃ to determine MICs.
MIC values for C. neoformans and the Candida strains were determined in 96-well plates using a broth serial dilution method in accordance with the Clinical and Laboratory Standards Institute guideline. Fluconazole was used as a reference standard, and the final concentrations of the test reagents and fluconazole ranged from 0.1 to 1,000 µg/ml and 0.125 to 256 µg/ml, respectively.
The optimal growth of the Malassezia species was obtained in LN medium, and the results presented were obtained from four independent experiments. Overall, similar MIC values were obtained in each independently performed in vitro susceptibility test.
The MIC values of the eight candidate extracts and two natural products against M. restricta and M. globosa are presented in Table 1. The Malassezia species showed susceptibility to five of the eight candidate extracts, i.e., the C. crenata shell, C. sinensis leaf, A. vulgaris, oil-soluble Glycyrrhiza, and A. membranaceus root extracts. Overall, M. restricta was more susceptible to most extracts than M. globosa.
The C. crenata shell, C. sinensis leaf, and oil-soluble Glycyrrhiza extracts had relatively low MIC values (≤0.5 mg/ml) against both strains. The C. crenata shell extract showed the highest antifungal activity against M. restricta among the eight candidate extracts, with the MIC values of 0.065 to 0.125 mg/ml. This was followed by the A. vulgaris, oil-soluble Glycyrrhiza, A. membranaceus root (MICs: 0.125 mg/ml), and C. sinensis leaf (MICs: 0.25 mg/ml) extracts. In comparison, for M. globosa, the MIC values of the oil-soluble Glycyrrhiza extract (0.25~0.5 mg/ml) were lower than those of the other extracts. The C. crenata shell and C. sinensis leaf extracts followed, with MIC values of 0.5 mg/ml.
Both strains showed in vitro resistance to the P. sylvestris leaf extract, P. mume fruit extract, Coix lacryma-jobi var. ma-yuen seed extract, dipotassium glycyrrhizate, and phytosphingosine. The MIC values of the A. vulgaris and A. membranaceus root extracts showed apparent differences in antifungal susceptibility between the two Malassezia species.
In the follow-up experiments, the C. crenata shell extract, which demonstrated the overall lowest MIC values against M. restricta (1st rank of 10 candidates) and M. globosa (2nd rank of 10 candidates), showed no activity against the other two Malassezia species, M. sympodialis and M. slooffiae. The oil-soluble Glycyrrhiza extract, the other candidate showing anti-Malassezia activity against M. restricta (2nd rank of 10 candidates) and M. globosa (1st rank of 10 candidates), also presented anti-Malassezia activity against M. sympodialis (MICs: 500 µg/ml) and M. slooffiae (MICs: 1,000 µg/ml). Ketoconazole showed activity against all tested Malassezia species, with lower MIC values ranging from 0.03 to 0.125 µg/ml (Table 2).
In the experiments with the other yeast organisms, C. neoformans, C. albicans, C. tropicalis, and C. parapsilosis, the C. crenata shell and oil-soluble Glycyrrhiza extracts also demonstrated antifungal activity. The MIC values were considerably lower than those obtained against the Malassezia species and ranged from 3.9 to 15.6 µg/ml with the C. crenata shell extract and from 6.25 to 50 µg/ml with the oil-soluble Glycyrrhiza extract. Fluconazole also showed activity (MICs: 2~8 µg/ml) against the Cryptococcus and three Candia strains (Table 3).
Oral and topical antifungal agents became widely used for treatment of dandruff and scalp SD following the confirmation of the etiological relationship mentioned previously1112. In several previous studies, the severity of the disease was positively correlated with the number of microorganisms and lesions, which were generally confined to areas of heavy colonization. During treatment with topical antifungal agents, the yeast load was reduced, and clinical improvement was observed. Furthermore, when the disease recurred, the yeasts were presented in greater numbers1314.
In the present study, overall, three candidate extracts, including the C. crenata shell, C. sinensis leaf, and oil-soluble Glycyrrhiza extracts, generated relatively low MIC values against both M. restricta and M. globosa. This suggests that the extracts have anti-Malassezia activities against the major Malassezia species associated with SD and may potentially be active components in shampoos for SD. It is interesting that the three active extracts demonstrated much higher antifungal activities than phytosphingosine, which has been commonly used in various cosmetic products because of its anti-inflammatory and antimicrobial potency. It has previously been reported that the growth inhibition of M. furfur could be achieved in vitro at extremely high phytosphingosine concentrations (mean MIC of 6,250 µg/ml), whereas C. albicans strains were inhibited at concentrations between 152 and 269 µg/ml15.
However, the results showed some differences among the extracts in their effects on the Malassezia species. The greatest anti-Malassezia activities were detected in the C. crenata shell extract against M. restricta and in the oil-soluble Glycyrrhiza extract against M. globosa. The results also showed apparent differences between the two Malassezia species in their susceptibility to the A. vulgaris and A. membranaceus root extracts. This indicates that different Malassezia species can have different susceptibilities, which implies the importance of in vitro susceptibility tests. Interestingly, the C. crenata shell extract, which demonstrated prominent activity against M. restricta and M. globosa, showed no activity against M. sympodialis and M. slooffiae. This suggests that the therapeutic activity of the C. crenata shell extract against major pathogens of scalp SD could be selective. As previously reported by Kim et al.16, M. restricta and M. globosa are predominant Malassezia species associated with scalp SD in East Asia, with a frequency of 60% to 100% for each species. In contrast, M. sympodialis was found in 11.1% of East Asian scalp SD patients, and M. slooffiae was not detected in the above study. The oil-soluble Glycyrrhiza extract presented the same or higher activity than the C. crenata shell extract against M. restricta and M. globosa in the follow-up experiment. This suggests that the oil-soluble Glycyrrhiza extract is compatible with the C. crenata shell extract as an active reagent of anti-SD formulations. The C. crenata shell extract and oil-soluble Glycyrrhiza extract also showed fungistatic activity against the other common facultative pathogenic yeasts, Cryptococcus and Candida. The results indicate the potential use of the extracts for other fungal infections. Both ketoconazole and fluconazole, commonly used antifungal agents, also showed positive inhibition, demonstrating that the assays were effective. Although various medicated and commercial shampoos with ketoconazole and zinc pyrithione have been on the market, patients have been repulsed by long-term exposure to these antifungal medications. As drug-resistant fungal infections have been on the rise over the past decade, plant sources increasingly attract more attention to provide new and more effective antifungal products17181920. Corticosteroids used to relieve scalp inflammation seem to be inappropriate for long-term and prophylactic use because of the chronic and relapsing nature of SD. Although steroids show efficacy and safety in short-term treatments, they could lead to frequent relapses shortly after the treatment discontinues2122. Shampoos containing plant extracts with anti-Malassezia activity would be an important part in a long-term treatment as an alternative and also for supportive management. In addition, it would be adequate to use them for prophylactic management in patients with mild dandruff or as a maintenance treatment to control SD. If the antifungal effects of the plant extracts are verified, their use would be able to improve the compliance of patients with an aversion to long-term antifungal treatment and steroid use. Compliance is one of the most important factors in the treatment of chronic conditions such as SD and dandruff.
However, this study has the following limitations: the first limitation is related to the interpretation of the results. We do not have sufficient information to evaluate the quality of the study. No reference MIC data for the studied plant extracts against Malassezia species have been reported and accumulated before. The second limitation is that we need clinical correlation studies.
Further studies are required to support our results. Above all, it is necessary to identify the actual active compounds in the extracts that are responsible for the antifungal activity. For example, in a previous study that investigated in vitro anti-Malassezia activity of Asparagus racemosus, Onlom et al.23 found through additional experiments that the high saponin content was associated with the antifungal activity. Analyses of interactions between the plant extracts, especially their active compounds, and several vehicles used in shampoo formulations should be performed from as many angles as possible. Vehicles may increase or decrease the activities of antifungal reagents depending on their solubility or chemical interactions. By extension, it would also be meaningful to explore synergistic or antagonistic interactions between the extracts and existing medications, such as ketoconazole and zinc pyrithione. Then, studies that can prove clinical correlations should be conducted to apply these results to clinical fields. The initial severity and characteristics of SD in individual patients should be evaluated, and clinical changes induced by a new formulation should be observed for a certain period. Potential complications and subjective satisfaction, which are important factors for patient compliance, should also be investigated in a series of clinical studies.
Despite the limitations of being a simple observational study with a relatively small sample size, this study is significant in that it reveals the potential of plant extracts, including the C. crenata shell and oil-soluble Glycyrrhiza extracts, to become active ingredients in anti-SD and -dandruff formulations. Few studies have been conducted to prove the anti-Malassezia activity of natural ingredients in shampoos by in vitro laboratory tests.
C. crenata is a species of chestnut. The inner shell of chestnut has been used topically as an anti-wrinkle and skin-firming agent for a long time in East Asia. It has also been reported that C. crenata shell extract has anti-inflammatory/anti-allergic activity, which inhibits mast cell degranulation24. However, no information on its antifungal activity has been previously published to our knowledge. The oil-soluble Glycyrrhiza (licorice) extract is a well-known herbal ingredient in various cosmetics for its skin whitening effects. Some reports have described the in vitro antimicrobial and antifungal activity of the oil-soluble Glycyrrhiza extract. Rohinishree and Negi25 reported inhibitory efficacy of licorice extract in controlling growth and pathogenicity of Staphylococcus aureus. Sato et al.26 described antifungal activity of the oil-soluble Glycyrrhiza extract against Arthrinium sacchari and Chaetomium funicola and raised the possibility of adding the preparation made from the extract to beverages in polyethylene terephthalate bottles. However, as the dermatologic field lacks scientific evidence for fungistatic activity of the oil-soluble Glycyrrhiza extract, little is known on its potential use for the treatment of skin disease. Especially, no research has been reported on its activity against Malassezia species associated with SD to our knowledge.
In conclusion, commercial or medicated shampoos containing C. crenata shell and oil-soluble Glycyrrhiza extracts could be a long-term treatment of choice for SD, although more advanced studies are required. The anti-inflammatory/-allergic activity of the extracts might also help to control symptoms of scalp SD. This study provides the foundation for further research to develop new anti-Malassezia shampoos with C. crenata shell and oil-soluble Glycyrrhiza extracts.
Figures and Tables
Table 1
Minimal inhibitory concentrations values (mg/ml) of 10 candidate extracts and natural products tested against Malassezia globosa and M. restricta
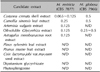
Table 2
Minimal inhibitory concentrations values (µg/ml) of Castanea crenata shell extract, oil-soluble Glycyrrhiza extract, and ketoconazole against Malassezia species

Table 3
Minimal inhibitory concentrations values (µg/ml) of Castanea crenata shell extract, oil-soluble Glycyrrhiza extract, and fluconazole against Cryptococcus and Candida species

References
1. Crespo Erchiga V, Delgado Florencio V. Malassezia species in skin diseases. Curr Opin Infect Dis. 2002; 15:133–142.

2. Gupta AK, Ryder JE, Nicol K, Cooper EA. Superficial fungal infections: an update on pityriasis versicolor, seborrheic dermatitis, tinea capitis, and onychomycosis. Clin Dermatol. 2003; 21:417–425.

3. Tajima M, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: comparison with other diseases and healthy subjects. J Invest Dermatol. 2008; 128:345–351.

4. Lee YW, Byun HJ, Kim BJ, Kim DH, Lim YY, Lee JW, et al. Distribution of malassezia species on the scalp in korean seborrheic dermatitis patients. Ann Dermatol. 2011; 23:156–161.

5. Guého E, Boekhout T, Ashbee HR, Guillot J, Van Belkum A, Faergemann J. The role of Malassezia species in the ecology of human skin and as pathogens. Med Mycol. 1998; 36:Suppl 1. 220–229.
6. Carrillo-Muñoz AJ, Rojas F, Tur-Tur C, de Los Ángeles Sosa M, Diez GO, Espada CM, et al. In vitro antifungal activity of topical and systemic antifungal drugs against Malassezia species. Mycoses. 2013; 56:571–575.

7. Turner GA, Matheson JR, Li GZ, Fei XQ, Zhu D, Baines FL. Enhanced efficacy and sensory properties of an anti-dandruff shampoo containing zinc pyrithione and climbazole. Int J Cosmet Sci. 2013; 35:78–83.

8. Kim YR, Kim JH, Shin HJ, Choe YB, Ahn KJ, Lee YW. Clinical evaluation of a new-formula shampoo for scalp seborrheic dermatitis containing extract of rosa centifolia petals and epigallocatechin gallate: a randomized, double-blind, controlled study. Ann Dermatol. 2014; 26:733–738.

9. Leeming JP, Notman FH. Improved methods for isolation and enumeration of Malassezia furfur from human skin. J Clin Microbiol. 1987; 25:2017–2019.

10. Sugita T, Tajima M, Ito T, Saito M, Tsuboi R, Nishikawa A. Antifungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J Clin Microbiol. 2005; 43:2824–2829.

11. Piérard-Franchimont C, Goffin V, Decroix J, Piérard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis. Skin Pharmacol Appl Skin Physiol. 2002; 15:434–441.

12. Ratnavel RC, Squire RA, Boorman GC. Clinical efficacies of shampoos containing ciclopirox olamine (1.5%) and ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis. J Dermatolog Treat. 2007; 18:88–96.

13. Arrese JE, Piérard-Franchimont C, De Doncker P, Heremans A, Cauwenbergh G, Piérard GE. Effect of ketoconazole-medicated shampoos on squamometry and Malassezia ovalis load in pityriasis capitis. Cutis. 1996; 58:235–237.
14. McGinley KJ, Leyden JJ, Marples RR, Kligman AM. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975; 64:401–405.

15. Nenoff P, Haustein UF. In vitro activity of phytosphingosines against Malassezia furfur and Candida albicans. Acta Derm Venereol. 2002; 82:170–173.

16. Kim SY, Kim SH, Kim SN, Kim AR, Kim YR, Kim MJ, et al. Isolation and identification of Malassezia species from Chinese and Korean patients with seborrheic dermatitis and in vitro studies on their bioactivity on sebaceous lipids and IL-8 production. Mycoses. 2016; 59:274–280.

17. Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996; 40:279–291.

18. Alexander BD, Perfect JR. Antifungal resistance trends towards the year 2000. Implications for therapy and new approaches. Drugs. 1997; 54:657–678.
19. Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998; 1:547–557.

20. Fatima A, Gupta VK, Luqman S, Negi AS, Kumar JK, Shanker K, et al. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother Res. 2009; 23:1190–1193.

21. Rigopoulos D, Ioannides D, Kalogeromitros D, Gregoriou S, Katsambas A. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis. A randomized open-label clinical trial. Br J Dermatol. 2004; 151:1071–1075.

23. Onlom C, Khanthawong S, Waranuch N, Ingkaninan K. In vitro anti-Malassezia activity and potential use in anti-dandruff formulation of Asparagus racemosus. Int J Cosmet Sci. 2014; 36:74–78.

24. Hahn HG, Oh HS, Cheon SH, Oak MH, Kim YR, Kim KM. Excavation of lead compounds that inhibit mast cell degranulation by combinatorial chemistry and activity-guided. Arch Pharm Res. 2004; 27:518–523.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download