Abstract
Background
Rhus verniciflua Stokes (RV) has traditionally been used in Korea as an indigenous food (Rhus chicken soup) and as an herbal medicinal plant. While the anticancer, antimicrobial, and anti-inflammatory properties of RV have been actively studied in the medical field, its antioxidant effects in the skin that resist the reactive oxygen species in keratinocytes and fibroblasts is less understood.
Objective
We designed to evaluate the effects of R. verniciflua Stokes extract (RVE) on the photo-aged skin by an in vitro experiment using human fibroblasts and an in vivo experiment using a photo-aged murine model.
Methods
For the in vitro experiments, human fibroblasts irradiated with ultraviolet (UV) B were treated with RVE or vehicle, and the growth levels and the expression level of type 1 procollagen were compared. For the in vivo experiment, photo-aged mice irradiated with UVB and UVA were administered drinking water with or without RVE, and histological changes and the expression level of type 1 procollagen and matrix metalloprotease (MMP)-13 were compared.
Results
In vitro experiments using fibroblasts irradiated with UVB showed that RVE promoted growth and significantly increased the expression of type 1 procollagen as compared to the control group. In the photo-aged mice, RVE increased collagen content in the dermis and promoted the synthesis of type 1 procollagen without any visible decrease in MMP-13 as compared to control group.
Aging of the skin includes intrinsic and extrinsic aging. Intrinsic aging occurs as the person ages, while extrinsic aging mainly involves photo-aging by ultraviolet (UV) exposure1. Gross changes caused by aging of the skin present as wrinkles, loss of elasticity, and increase of pigmentation. These changes result from a decrease in collagen and elastin produced from fibroblasts and abnormal melanocyte pigment production. For the prevention or treatment of skin aging, a wide range of studies have been conducted with the aim to increase synthesis of collagen and elastin as well as prevent their loss, which were targeted to block UV radiation and develop antioxidants2.
Rhus verniciflua Stokes (RV) is a tree belonging to the Anacardiaceae family. It mainly grows in East Asian countries, such as Korea, China, and Japan. In Korea, it has long been used as an ingredient in oriental medicine and traditional food. Various studies conducted on RV have reported its anticancer, antimicrobial, and anti-inflammatory effects 3456. In addition, R. verniciflua Stokes extract (RVE) contains a diverse range of polyphenols, which have been reported to present antioxidant effects357. When keratinocytes were treated with RVE, the polyphenols were shown to increase resistance to oxidative stress and matrix metalloprotease (MMP)-13 expression, which are associated with aging, and showed a decrease in dermal fibroblasts. These results indicate the possible use of RVE as a substance to prevent the skin aging process8. While some in vitro studies related to aging have reported the suppression of MMP-13 due to the antioxidant effects of the RVE, there have been no in vivo studies presenting the anti-aging effects of RVE on the skin.
Therefore, we performed this study to elucidate whether RVE prevents photo-aging in skin. An in vitro experiment was carried out with cultured fibroblasts, the major cell type related to skin aging, and an in vivo experiment was carried out using a photo-aged murine model that was induced by repeated UV irradiation.
The RVE water used in this experiment was prepared by heating the tree in water to eliminate urushiol and other toxic chemicals that cause allergic contact dermatitis. Briefly, naturally grown RV was obtained from Wonju, Korea. For detoxification of the RV samples during the RVE preparation process, Kim's method was employed9. The stem bark of dried RV (50 g) was soaked and autoclaved in 1 L of water at 125℃ for 3 hours. The water extract was then filtered with a glass fiber filter (extra thick; Gelman Sciences, Ann Arbor, MI, USA), and the filtered solution was used as the sample. RVE water has been reported to contain strong antioxidant phenolic compounds, such as gallic acid, butin, butein, fisetin, quercetin, and sulfuretin as the major components1011.
To evaluate the effect of RVE on fibroblasts, which are a part of the dermis and synthesize collagen and the extracellular matrix, we cultured primary fibroblasts detached from human foreskin and used 3~7 passage cells. These cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 100 U/ml penicillin and 100 µg/ml streptomycin in a CO2 incubator at 37℃. Exponential phase cells were then subcultured using 0.025% trypsin-ethylenediaminetetraacetic acid.
To assess the cytotoxicity of RVE on cultured fibroblasts, we measured cell count using the MTT assay. The concentrations of RVE ranged from 0.01% to 100%, and a group containing no RVE was used as the control group. The MTT assay was repeated five times and the average value was recorded. For the effects of RVE on collagen synthesis, real time quantitative reverse transcriptase polymerase chain reaction (quantitative RT-PCR) was performed.
To assess the cytotoxicity of RVE on cultured fibroblasts exposed to UV, we divided 1×104 cells into 35 mm plastic culture dishes and cultured them for an additional 24 hours. The cells were then irradiated with 50 mJ/cm2 UVB (Philips TL20W/12 RS lamps, CLEO; Philips, Amsterdam, Netherlands) and cultured in the medium with 0% to 100% diluted RVE for 24 hours. Alternatively, the control group was irradiated with a sham light (fluorescent light) and cultured in the medium with 0% to 100% diluted RVE for 24 hours before MTT assays.
To assess collagen synthesis in fibroblasts under UV exposure, 1×106 cells were divided in 60 mm plastic culture dishes, cultured for 24 hours, and then irradiated with 50 mJ/cm2 UVB light. For the control groups, cells were cultured in the medium containing no RVE or the media containing 0.01% to 10% concentrations of RVE for 24 hours. Quantitative RT-PCR was then performed in both the treated and the control groups for comparison of type 1 procollagen mRNA levels. The primer sequences are as follows: GAPDH 5′-TGAGCTGAACGGGAAG-3′, and 5′-CTGTAGCCAAATTCGTTGT-3; Procollagen type1-a1 5′-TCAGGTGAGCAGGATGTGAG-3′, and 5′-GCTTTGGGAGCTTCAGTGAC-3′.
The Yonsei University Wonju College of Medicine Institutional Animal Care and Use Committee (IACUC), Wonju, Korea approved all animal procedures. Female hairless mice (h/h) aged 22~24 weeks were prepared and divided into the following groups: the RVE treated group (UV light+RVE water) (n=7) was irradiated with UV light to induce photo-aging and fed with drinking water containing RVE. The water control group (UV light+no RVE water) (n=7) was irradiated with UV light to induce photo-aging and fed with drinking water lacking RVE. The normal control group (sham light+no RVE water) (n=6) was irradiated with the sham light (fluorescent light) and fed with drinking water lacking RVE.
In order to induce photo-aging, the mice were irradiated with UVA (14 J/cm2) and UVB (40 mJ/cm2) three times a week for 8 weeks as previously reported12. The UVA and UVB lamps used were Philips 40W lamps (CLEO) and Philips TL20W/12 RS lamps (CLEO) respectively. The fluorescent light irradiation, which was used as a control light source for comparison with the UV light, was conducted in the same manner as the UV irradiation. For drinking water, a 1:1 diluted solution of RVE and tap water was administered to the RVE treated group every day for 8 weeks, and only tap water was administered to the water control group every day for 8 weeks. To assess the effect of RVE on photo-aging skin, skin tissue was taken stained with H&E for dermal thickness, Masson-trichrome stain for collagen density. In addition, Western blotting was carried out to assess collagen synthesis and collagenase.
Each value is indicated as the mean±standard error. In vitro data were analyzed using the Mann-Whitney U-test and in vivo data were analyzed using the one way ANOVA. Statistical analysis was performed using PASW ver. 18.0 (IBM Co., Armonk, NY, USA), and p-values <0.05 were considered statistically significant.
To assess the effect of RVE on fibroblasts, RVE at varying concentrations (ranging from 0.01%~100%) was added to the cultured fibroblasts with or without UV light irradiation. Negligible effects were observed at the 0.01% to 1.0% concentration of RVE. However, at 5% and 10% RVE concentrations, the fibroblast count increased by 8% and 12% relative to the control, respectively. On the other hand, at 50% and 100% RVE concentrations, cytotoxicity was observed (Fig. 1A). When irradiated with UV, cell growth was confirmed up to RVE concentration levels of 10%, but cytotoxicity was observed at 50% and 100% concentration levels (Fig. 1B).
The valid concentration was set at 10% based on MTT assay results, and then the effect of RVE on collagen synthesis was assessed according to RVE concentration. The results of quantitative RT-PCR showed that type 1 procollagen mRNA was increased relative to the control at all RVE concentrations. In particular, 0.05%, 0.5%, and 1% RVE increased the levels significantly (Fig. 2A). When irradiated with UVB, it was also found that the expression of type 1 procollagen mRNA increased at RVE concentration levels of 0.01%, 0.1%, and 1%, proportionally to the concentration level (Fig. 2B).
Histological findings based on H&E showed that the dermal thickness in the RVE treated group (UV light+RVE water) was significantly increased compared to the water control group (UV light+no RVE water) and the normal control group (sham light+no RVE water) (Fig. 3A, C). Masson's trichrome staining showed that the collagen density was significantly increased in the RVE treated group compared to the water control group and the normal control group (Fig. 3B, D).
Western blotting showed significant differences in the production of type 1 procollagen between the RVE treated group and the water control group, but similar production levels were seen between the RVE treated group and the normal control group. On the other hand, there were no differences in MMP-13 expression among the RVE treated group, the water control group, and the normal control group (Fig. 4).
Skin aging includes intrinsic aging, which occurs as the person ages, and photo-aging, which occurs on UV light exposed areas, such as the face, neck, and the back of the hands. Histologically, photo-aging presents as a decrease in epidermal thickness compared to intrinsic aging, and causes cellular dysplasia and a decrease in Langerhans cells in the epidermis. In the dermis, photo-aging significantly decreases the amount of elastin and collagen and further promotes the production of MMPs including collagenase and causes the generation and infiltration of various inflammatory cells. Also, photo-aging has been reported to decrease transforming growth factor (TGF)-beta receptors and procollagen synthesis due to alterations of the AP-1 signal, but was shown to increase MMP activity 13141516.
Most therapeutic or preventive modalities for skin aging decrease the expression of MMP to prevent the destruction of collagen and elastin and promote the synthesis of collagen. Others include UV blockers to prevent photo-aging17. Topical retinoids or alpha-hydroxyacids, chemical peels, and ablative lasers have also been used to promote collagen synthesis and remodel the dermal matrix1819202122. A wide range of natural substances have also been used2324. Polyphenol compounds are secondary metabolites found in a variety of plants, which are biologically active substances that can be obtained in large quantities at low cost252627. RV has long been widely used in traditional foods and folk medicines in East Asian countries including Korea, China, and Japan. However, urushiol is a toxic substance contained in RV that causes serious side effects, such as allergic contact dermatitis, which has contributed to many constraints in medical research.
Urushiol in low concentrations has a non-toxic reaction on normal cells but suppresses the growth of cancer cells. With the recent development of a detoxication method, studies have been carried out to determine the anti-inflammatory and anticancer effects of various flavonoids and polyphenol compounds extracted from RV36282930. Therefore, we used urushiol-free RVE and first performed MTT assays to assess cytotoxicity at varying RVE concentrations. Cytotoxicity of RVE in vitro was revealed at high concentrations, such as 50% and 100%. The growth of fibroblasts irradiated with UV was observed up to RVE concentration levels of 10%, but cytotoxicity occurred at 50% and 100%. Also, RVE treatment increased the synthesis of type 1 procollagen in fibroblasts. This observation elucidated that RVE inhibits the suppression of fibroblast proliferation induced by UVB. On the other hand, UV irradiation with RVE treatment led to an increased fibroblast proliferation compared to RVE treatment alone. In the previous study, expression of the various flavonoids and antioxidant enzymes contained in the RV rather increased in the stressful situation31. It suggested that the stress caused by UV promotes the activities of RV, possibly stimulating the antiaging potential unlike the control group. Flavonoids have been known to affect the fibroblast and its production of collagen, so various flavonoids in the RV would affect the proliferation of fibroblasts and the generation of the collagen32. We also inferred from previous reports that RVE has a protective effect on human fibroblasts against oxidative stress due to UV radiation and that various polyphenols contained in RVE increase resistance against reactive oxygen species (ROS)8.
In order to evaluate the effect of RVE on photo-aged skin, we used drinking water containing RVE in animal experiments because RV has traditionally been ingested as an additive in foods and drinks for human health. We measured dermal thickness and dermal collagen density to evaluate the anti-aging effects of RVE and the production of type 1 procollagen and MMP-13 to understand its action mechanism. Most skin collagen is type 1, and type 1 procollagen is produced as a precursor of collagen and becomes a component of the dermis33. The content of dermal collagen is maintained between its synthesis in the fibroblasts and breakdown by collagenases, such as MMP-1 in humans and MMP-13 in mice34. With aging, collagen synthesis decreases but MMP increases, speeding up the decrease of collagen density. Particularly in photo-aging, UV increases the generation of ROS, which activates AP-1. In this study, we observed that dermal thickness in the RVE treated group (UV light+RVE water) was considerably increased relative to the water control group (UV light+no RVE water) or the normal control group (sham light+no RVE water).
Increased expression of MMP-1, -3, and -9 by UV, which promote the breakdown of collagen in human skin, is a major biomarker in photo-aging research. MMP-1 can be functionally substituted by measuring MMP-13, which breaks down collagen in mice35. Although mice administered RVE showed a slight decrease in MMP-13, there were no significant differences among the three treatment groups. On the other hand, the amount of type 1 procollagen increased significantly. Liu et al.8 reported increased resistance to H2O2 and decreased MMP-1 levels in an experiment using human fibroblasts treated with RVE. However, our in vivo results show a prevention of photo-aging through the increased synthesis of collagen rather than the suppression of collagen breakdown by decreasdecreasing MMP levels. These results may be explained by previously reported mechanism of the interaction of phenolic compounds, such as gallic acid contained in RVE and collagen scaffolds enhances collagen structure stability and suppresses collagenase activity36. In this experiment, it is assumed that RVE restores the suppressed synthesis of collagen, and maintains the stability of collagen. On the other hand, royal jelly and cinnamon extract, known as natural anti-aging substances, have been reported to have anti-aging effects by stimulating various signaling pathways that promote the synthesis of collagen, such as TGF-beta and insulin-like growth factor-1 in fibroblasts2437. While RVE is thought to be involved in collagen synthesis in a similar manner, further research will be necessary to ascertain this. A limitation of this study is that we did not include a direct verification of the active ingredients.
In conclusion, this study presents the possibility of RVE in the prevention of skin aging, especially photo-aging, through its ability to improve the growth and function of dermal fibroblasts increasing collagen synthesis.
Figures and Tables
 | Fig. 1Rhus verniciflua Stokes extract (RVE) stimulated the proliferation of human dermal fibroblasts with or without ultraviolet (UV) irradiation in vitro. Cultured human primary dermal fibroblasts were exposed to various concentrations of RVE (0%~100%) and evaluated by MTT assay. (A) Negligible effects were observed at the 0.01% to 1.00% RVE concentrations; however, the cell count increased by 8% and 12% relative to the control at 5% and 10% RVE concentrations, respectively. In 50% and 100% RVE concentrations, cytotoxicity was observed. (B) When irradiated with UV, cell growth was observed up to RVE concentration levels of 10%, but cytotoxicity occurred at 50% and 100%. Normal control (NC) had no RVE treatment and no UV irradiation. The values used are the mean±standard error (SE) from 96 wells (A) and 12 wells (B), respectively. *p<0.05, **p<0.01 compared to the control group (0% RVE). |
 | Fig. 2Rhus verniciflua Stokes extract (RVE) increased expression of type 1 procollagen mRNA in human dermal fibroblasts without or with ultraviolet (UV) irradiation in vitro. Cultured human primary dermal fibroblasts were treated with various concentrations of RVE (0%~10%) and evaluated by quantitative reverse transcriptase polymerase chain reaction. (A) The type 1 procollagen mRNA levels were increased at all RVE concentrations. In particular, 0,1%, 0.05%, 0.5%, and 1.0% RVE increased levels significantly. (B) When irradiated with UVB, the type 1 procollagen mRNA increased at RVE concentration levels of 0.01%, 0.05%, 0.1%, and 1%, proportionally to the concentration level. Values shown are the mean±standard deviation (SD) from 60 mm dishes. *p<0.05, **p<0.01 compared to the control group (0% RVE). |
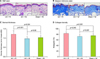 | Fig. 3Rhus verniciflua Stokes extract (RVE) increased dermal thickness and collagen density in photo-aged murine skin. The dermal effects of RVE were evaluated by measuring dermal thickness (C) using H&E staining, ×100 (A). Dermal thickness was defined as the distance from the subcutaneous fat to the dermo-epidermal junction. To measure collagen density (D), we performed Masson trichrome staining, ×100 (B). We used the histogram function of Photoshop software to evaluate collagen density. RVE significantly increased collagen density compared to the water only control. UV+R: RVE treated group (UV light+RVE water), UV+W: water control group (UV light+no RVE water), Sham+W: normal control group (Sham light+no RVE water). UV: ultraviolet light irradiation, Sham: sham light irradiation, W: water supply, R: R. verniciflua Stokes extracts supply. |
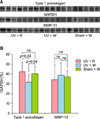 | Fig. 4Rhus verniciflua Stokes extract (RVE) affected type 1 procollagen but not matrix metalloprotease (MMP)-13 in photo-aged murine skin. (A) Based on Western blot analysis, (B) RVE increased the production of type 1 procollagen collagen up to the level of the normal control group but did not affect MMP-13 levels. UV+R: RVE treated group (UV light+RVE water), UV+W: water control group (UV light+no RVE water), Sham+W: normal control group (Sham light+no RVE water). GAPDH: glyceraldehyde 3-phosphate dehydrogenase, UV: ultraviolet light irradiation, Sham: sham light irradiation, W: water supply, R: R. verniciflua Stokes extracts supply. |
ACKNOWLEDGMENT
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. HN12C0061).
References
1. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002; 138:1462–1470.

3. Kim JH, Shin YC, Ko SG. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by Rhus verniciflua Stokes. Mediators Inflamm. 2014; 2014:154561.
4. Kitts DD, Lim KT. Antitumorigenic and cytotoxic properties of an ethanol extract derived from Rhus verniciflua Stokes (RVS). J Toxicol Environ Health A. 2001; 64:357–371.

5. Lim KT, Hu C, Kitts DD. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem Toxicol. 2001; 39:229–237.

6. Suk KT, Baik SK, Kim HS, Park SM, Paeng KJ, Uh Y, et al. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter. 2011; 16:434–443.

7. Chen H, Wang C, Ye J, Zhou H, Yuan J. Phenolic extracts from Rhus verniciflua Stokes bark by decompressing inner ebullition and their antioxidant activities. Nat Prod Res. 2014; 28:496–499.

8. Liu CS, Nam TG, Han MW, Ahn SM, Choi HS, Kim TY, et al. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci Biotechnol Biochem. 2013; 77:1682–1688.

9. Kim JB. Method for detoxification of the extracts from Rhus verniciflua. Korea patent;1999.
10. Kim IW, Shin DH, Baek NI. Identification of antioxidative components from ethanol extract of Rhus verniciflua Stokes. Korea J Food Sci Technol. 1999; 31:1654–1660.
11. Kim JB. Identification of antioxidative component from stem bark of Rhus verniciflua. Korea J Food Nutr. 2003; 16:60–65.
12. Park HY, Youm JK, Kwon MJ, Park BD, Lee SH, Choi EH. K6PC-5, a novel sphingosine kinase activator, improves long-term ultraviolet light-exposed aged murine skin. Exp Dermatol. 2008; 17:829–836.

13. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006; 55:1–19.

14. Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002; 1:705–720.

15. Tanaka H, Yamaba H, Kosugi N, Mizutani H, Nakata S. Fermentable metabolite of Zymomonas mobilis controls collagen reduction in photoaging skin by improving TGF-beta/Smad signaling suppression. Arch Dermatol Res. 2008; 300:Suppl 1. S57–S64.
16. Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008; 7:2 Suppl. s12–s16.
17. Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed. 2014; 30:55–61.

18. Alexiades-Armenakas M, Sarnoff D, Gotkin R, Sadick N. Multi-center clinical study and review of fractional ablative CO2 laser resurfacing for the treatment of rhytides, photoaging, scars and striae. J Drugs Dermatol. 2011; 10:352–362.
19. Dierickx CC, Anderson RR. Visible light treatment of photoaging. Dermatol Ther. 2005; 18:191–208.

20. Griffiths CE, Goldfarb MT, Finkel LJ, Roulia V, Bonawitz M, Hamilton TA, et al. Topical tretinoin (retinoic acid) treatment of hyperpigmented lesions associated with photoaging in Chinese and Japanese patients: a vehicle-controlled trial. J Am Acad Dermatol. 1994; 30:76–84.

21. Stern RS. Clinical practice. Treatment of photoaging. N Engl J Med. 2004; 350:1526–1534.
22. Van Scott EJ, Ditre CM, Yu RJ. Alpha-hydroxyacids in the treatment of signs of photoaging. Clin Dermatol. 1996; 14:217–226.

23. Roh SS, Lee MH, Hwang YL, Song HH, Jin MH, Park SG, et al. Stimulation of the extracellular matrix production in dermal fibroblasts by velvet antler extract. Ann Dermatol. 2010; 22:173–179.

24. Park HM, Hwang E, Lee KG, Han SM, Cho Y, Kim SY. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J Med Food. 2011; 14:899–906.

25. Chiang HM, Chen HC, Lin TJ, Shih IC, Wen KC. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem Toxicol. 2012; 50:4260–4269.

26. Furumura M, Sato N, Kusaba N, Takagaki K, Nakayama J. Oral administration of French maritime pine bark extract (Flavangenol(®)) improves clinical symptoms in photoaged facial skin. Clin Interv Aging. 2012; 7:275–286.
27. OyetakinWhite P, Tribout H, Baron E. Protective mechanisms of green tea polyphenols in skin. Oxid Med Cell Longev. 2012; 2012:560682.

28. Lee J, Chae J, Lee S, Kim K, Eo W, Kim S, et al. The efficacy and safety of standardized allergen-removed Rhus verniciflua extract as maintenance therapy after first-line chemotherapy in patients with advanced non-small cell lung cancer. Am J Chin Med. 2013; 41:773–787.

29. Lee SK, Jung HS, Eo WK, Lee SY, Kim SH, Shim BS. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: report of two cases. Ann Oncol. 2010; 21:1383–1385.

30. Sapkota K, Kim S, Park SE, Kim SJ. Detoxified extract of Rhus verniciflua stokes inhibits rotenone-induced apoptosis in human dopaminergic cells, SH-SY5Y. Cell Mol Neurobiol. 2011; 31:213–223.

31. Ko JH, Lee SJ, Lim KT. Rhus verniciflua Stokes glycoprotein (36kDa) has protective activity on carbon tetrachloride-induced liver injury in mice. Environ Toxicol Pharmacol. 2006; 22:8–14.

32. Stipcevic T, Piljac J, Vanden Berghe D. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum Nutr. 2006; 61:29–34.

33. Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000; 114:480–486.

34. Watanabe H, Shimizu T, Nishihira J, Abe R, Nakayama T, Taniguchi M, et al. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J Biol Chem. 2004; 279:1676–1683.

35. Kim HH, Lee MJ, Lee SR, Kim KH, Cho KH, Eun HC, et al. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech Ageing Dev. 2005; 126:1170–1177.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download