INTRODUCTION
Androgenetic alopecia (AGA), also known as pattern hair loss, is a relatively common disease in adults over 40 years old, with a prevalence of up to 73% in the general Asian population
1.
Although AGA itself does not cause serious health-related issues, it may cause personal, social, and work-related problems, all of which can affect life quality
23. Thus, AGA should not be regarded simply as an isolated disease and treatments should be holistic and comprehensive.
Miniaturization of the hair follicles and changes in hair cycle dynamics are crucial elements of AGA pathogenesis. Unlike male pattern hair loss, female pattern hair loss (FPHL) is characterized by a reduction in the number of terminal fibers per follicular unit over the crown and frontal scalp with retention of the frontal hairline
4. Various treatments have been used for AGA. The medical therapies commonly used in FPHL, in addition to topical minoxidil solution, are cyproterone acetate, spironolactone and flutamide, finasteride, topical 17α-estradiol (alfa-tradiol), mineral supplements, and prostaglandin analogs, as well as surgical therapy and light therapy
5678910.
However, combination therapies are often preferred in clinical settings, since they are expected to yield superior treatment outcomes compared with single agent regimens. The aim of the current study was to determine the efficacy of a commonly used combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil in Korean patients with FPHL.
Go to :

MATERIALS AND METHODS
Study design and subjects
This was a retrospective, noncomparative, single institution study. Patients diagnosed with FPHL between March 2010 and December 2015 at Wonju Severance Christian Hospital who had concurrently applied minoxidil and 17α-estradiol for more than 6 months were considered. Of the patients who visited our institution with FPHL, only patients with both gross photos and phototrichograms, before and after treatment, were enrolled. The patient age range was 18 to 71 years old and all patients were diagnosed with F type FPHL according to the basic and specific (BASP) classification
11. Patients with any dermatological disorder, systemic disease, or abnormal laboratory finding that required additional treatment and evaluation that could have affected the results of the study were excluded.
In addition to age, family history was also obtained, and the patients were categorized and stratified according to the severity indices suggested by the BASP classification scheme.
In the BASP classification nomenclature
11, LF1 is defined as the mild group, LF2 as the moderate group, and LF3 as the severe group.
This study was approved by the Institutional Review Board of Wonju College of Medicine, Yonsei University (IRB no. CR316011).
Drug treatment
For treatment, 0.025% 17α-estradiol (Ell-Cranell® alpha 0.025%; Galderma Korea, Co., Seoul, Korea) and 3% minoxidil (Minoxyl® 3%; Hyundai Pharm, Co., Seoul, Korea) were used. Using a predosed applicator, 3% minoxidil and 17α-estradiol were applied once a day (1 ml/application and 3 ml/application, respectively), and the head was massaged for approximately one minute to facilitate drug absorption. Topical 17α-estradiol was applied in the morning, whereas minoxidil was applied in the evening.
Efficacy evaluation
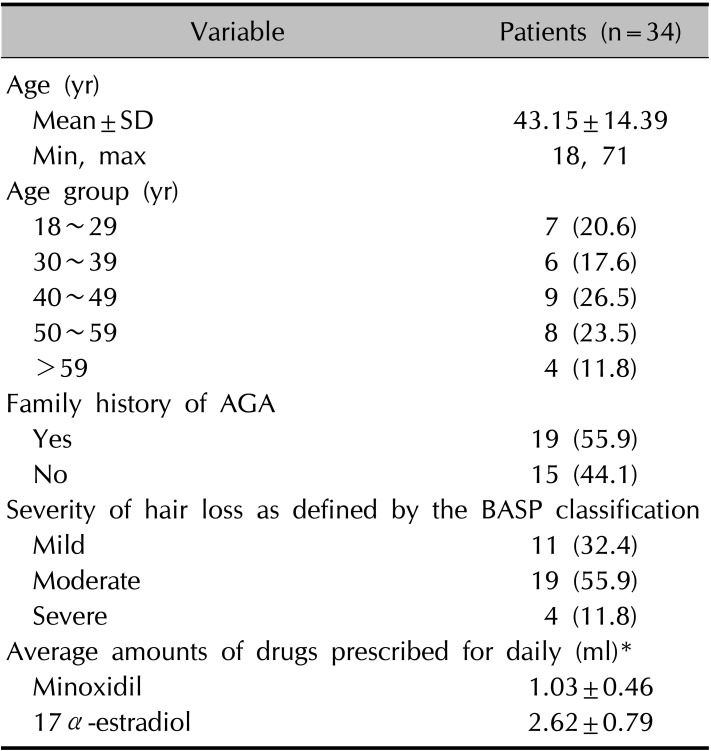
All subjects used the 0.025% 17α-estradiol and 3% minoxidil combination for 6 to 12 months. Efficacy was first evaluated by examining the change in the number of hairs per unit area as assessed by phototrichogram analysis at 6 months using a Folliscope® instrument (Lead M Co., Seoul, Korea).
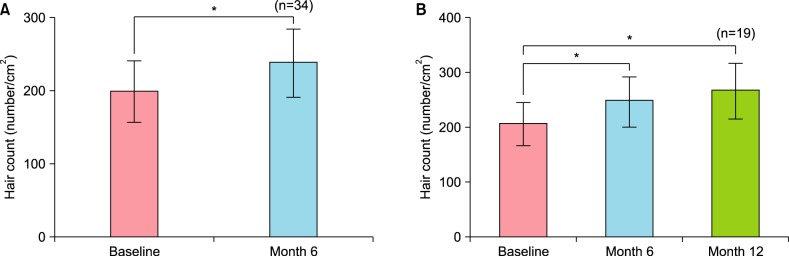
For the second efficacy evaluation, the change in the number of hairs per unit area after 12 months since the initial application and the changes in hair thickness at 6 months and 12 months were assessed.
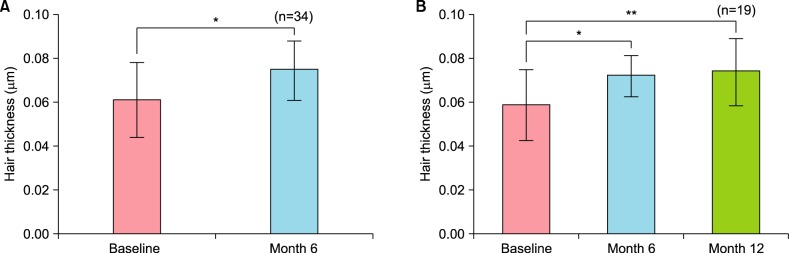
The subjects were subdivided according to family history and the changes of hair count at 6 months were compared between the two groups. The results were also compared among the groups stratified according to FPHL severity.
Clinical photograph evaluation
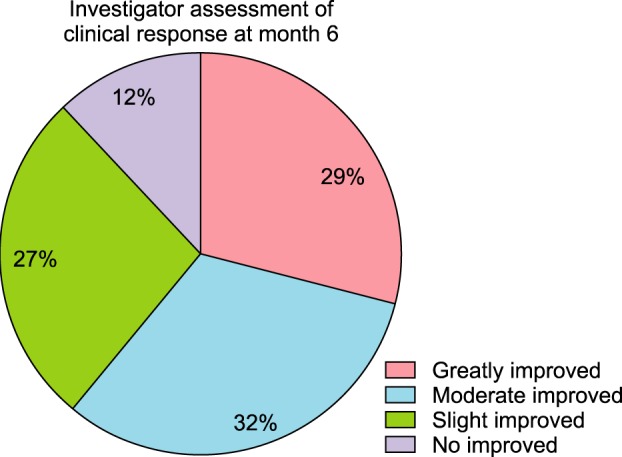
Prior to photographic documentation, each patient’s hair was combed in order to expose all areas of hair loss. Paired baseline photos were analyzed and compared with paired photos taken at 6 months or one year since the beginning of treatment. Photographs were evaluated on a scale ranging from ‘greatly improved’ to ‘not improved.’ The classifications were defined as follows: ‘greatly improved,’ more than 75% improvement; ‘moderately improved,’ 50%~75% growth; ‘slightly improved,’ 25%~50% improvement; and ‘not improved,’ less than 25% improvement.
Evaluation by phototrichogram analysis
The changes in hair thickness and density after 6 and 12 months of application were assessed by phototrichogram analysis using constant contrast and exposure.
Even though we had not done tattooing on the scalp, we had tried to improve the accuracy of taking phototrichogram from the same area by using the device developed by Canfield Scientific Inc. (Fairfield, NJ, USA), which could fix the forehead and chin.
The number of total hairs in a unit area (hair density) was measured by calculating the number of all hairs within the 70 mm2 circle. The diameters of the thickest five hairs were measured using a 200× lens and their average is presented as the average hair thickness.
Statistical analysis
Changes in the number of hairs and the hair diameters were assessed by phototrichogram analysis. Differences in data were analyzed by the paired t-test or the Wilcoxon signed rank test. Unless stated otherwise, the significance level for statistical analysis was 0.05. All data were analyzed with the SPSS statistics program ver. 23.0 (IBM Co., Armonk, NY, USA).
Go to :

DISCUSSION
AGA in females is also called FPHL. Compared with male pattern hair loss, FPHL tends to present in relatively less severe forms. However, these cosmetic issues still may seriously affect the quality of life
23.
Various medical therapies and surgical procedures are currently used in the treatment of FPHL. However, only limited medications are available with proven efficacy, and those that are found to be effective may not always result in satisfactory treatment outcomes.
Minoxidil for FPHL has been proven to be effective in numerous studies
1213.
The efficacy of this medication was also confirmed in recent meta-analyses in both sexes, which reported a large amount of supporting evidence
14.
However, the pathophysiology of minoxidil is still not fully understood. Minoxidil is converted to minoxidil sulfate after application, which is believed to shorten the telogen phase and cause the hair follicle to enter the anagen stage. Some evidence also suggests that minoxidil prolongs anagen and increases hair follicle size. In
in vitro studies of various skin and hair follicle cell types, minoxidil has been shown to stimulate cell proliferation, enhance vascular endothelial growth factor and prostaglandin synthesis, and inhibit collagen synthesis, all of which may be relevant to hair growth
15.
Minoxidil may also promote hair growth via its action as an adenosine triphosphate-sensitive potassium channel opener, which in turn causes relaxation of vascular smooth muscle, leading to increased cutaneous blood flow
16.
Recent studies have suggested that minoxidil also enhances hair growth by increasing the production of prostaglandin E2 through stimulation of prostaglandin endoperoxide synthase-1
17.
Besides minoxidil, 17α-estradiol has also been commonly used in Europe, South America, and Korea for the past decades.
17α-estradiol is a stereoisomer of the female hormone 17β-estradiol, which suppresses 5α-reductase activity and inhibits 17β-dehydrogenase activity. This inhibition slows the conversion of androstenedione to testosterone and increases the conversion of testosterone to 17β-estradiol and the conversion of androstendione to estrone, thus improving hair growth. However, 17α-estradiol does not exhibit estrogen activity or exhibit only very weak activity
181920. Kim et al.
21 reported that the hair count and thickness steadily increased after application of topical 17α estradiol when compared with the baseline.
In a double-blind randomized controlled trial by Orfanos and Vogels
22, 63% of all patients treated with 17α-estradiol had decreased telogen hair, which was comparable with the level (37%) in the control group.
Due to study by Wozel et al.
23, alfa-tradiol is involved more in stabilization or deceleration of hair loss, which is consistent with the results found in the previous placebo-controlled study of 51 patients (9 women) over 6 months by Van Neste and Rushton
24.
Combinations of medications with different mechanisms are broadly used in many medical fields in anticipation of the possibility of achieving greater efficacy than with single medications.
Since this was a noncomparative study, however, our data do not provide definitive information as to whether single use of either minoxidil or 17α-estradiol is more effective than the combination.
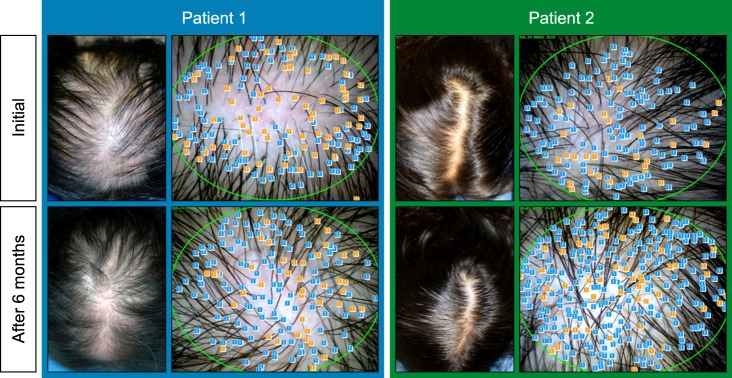
After 6 months of application, the majority of patients exhibited increased hair count and increased hair thickness. Also, hair count and hair thickness both constantly increased in patients who had continuously applied the medication for 12 months. These changes were also conspicuously observable with the naked eye (
Fig. 4).
Furthermore, after 6 months of application, most of patients with or without a family history and with mild type FPHL or moderate type FPHL exhibited increased hair count. When we compare the differences between patients with or without a family history and between patients with mild type FPHL or moderate type FPHL, both of them were not statistically significant. Taken together, combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil shows overall clinical efficacy regardless of symptom severity or family history. In addition to the satisfactory clinical efficacy observed during this study, no major side effects were observed in any patient.
As mentioned previously, treatment with minoxidil can induce an increase in hair density and hair thickness, whereas treatment with 17α-estradiol results in deceleration or stabilization of hair loss. The different mechanisms of the two medications may have contributed to the high effectiveness observed here.
Nonetheless, the clinical photos and phototrichograms had not been always performed on the same areas due to the absence of tattooing on the scalp. And the data were reviewed retrospectively, which may have created some limitations with respect to comparing the efficacy.
Additionally, we have to consider seasonal variations such as increase of hair follicle number in spring season; however, our patients were enrolled randomly regardless of season and majority of patients were not enrolled at spring season. So we assumed that the influence of seasonal variation was insignificant.
Further controlled studies are needed to definitively determine the effectiveness of the simultaneous use of these two medications.
Despite these limitations of our study, we found that the combination therapy of topical 0.025% 17α-estradiol and topical 3% minoxidil had clear clinical efficacy. Thus, this combination therapy is an effective and safe treatment modality for FPHL that can be used promptly in a clinical setting.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download