Abstract
Background
Periostin is a novel matricellular protein expressed in many tissues, including bone, periodontal ligament, and skin. Although its expression is prominent in various fibrotic conditions, studies of periostin in localized scleroderma are rare.
Methods
A retrospective study of 14 patients with confirmed mature stage localized scleroderma was undertaken. Fourteen age-matched and biopsy site-matched subjects with normal skin were included as controls. Collagen fiber deposition, periostin, procollagen, transforming growth factor-β, and matrix metalloproteinase (MMP)-1 expression were assessed and compared between the two groups. Co-localization of α-smooth muscle actin and periostin was evaluated using confocal microscopy.
Results
Periostin was predominantly expressed along the dermo-epidermal junction in the controls. Conversely, patients with localized scleroderma demonstrated increased collagen fiber deposition and periostin expression that was more widely distributed along the entire dermis. MMP-1 staining showed increased expression in the epidermis and dermis of patients compared to scanty expression in the controls. A semi-quantitative evaluation showed a higher proportion of excessive collagen bundle deposition (57.1% vs. 7.1%, p=0.013), diffuse periostin positivity (42.9% vs. 0%, p=0.016), and moderate MMP-1 positivity (71.4% vs. 7.1%, p=0.001) in patients than in the controls.
Periostin was first identified in 1993 as an 811-amino-acid protein secreted by murine osteoblasts, and it was originally termed osteoblast specific factor-21. It was later renamed periostin because of its localized expression in the periosteum and periodontal ligament2. However, many studies have confirmed the expression of periostin in various collagen-rich tissues, including the skin, and periostin is considered a matricellular protein3. Periostin has also been found in many pathologic conditions such as muscle injury, vascular injury, myocardial infarction, ovarian cancer, and colorectal cancer3. Furthermore, its expression is prominent in fibrotic conditions, including bone marrow fibrosis4. However, human studies of periostin in skin diseases are very rare.
Localized scleroderma is a chronic connective tissue disease characterized by excessive extracellular matrix (ECM) deposition, especially types I and III collagen, and consequent skin fibrosis. ECM accumulation in tissue represents an imbalance between the synthesis and degradation of ECM components. Matrix metalloproteinases (MMP) are a family of proteinases that are responsible for the degradation of ECM components, and MMP-1 can degrade types I, II, and III collagen. In vitro data from previous studies have shown that MMP-1 expression is decreased in the fibroblast of localized scleroderma and systemic sclerosis56. Furthermore, some in vivo data have shown an association between anti-MMP-1 autoantibody and scleroderma78, but human studies examining MMP-1 expression in scleroderma skin tissue are sparse. In addition to MMP-1, other molecules have been proven to be associated with the pathogenesis of scleroderma, including the transforming growth factor (TGF)-β19, which has been regarded as a key molecule, connective tissue growth factor (CTGF)10, and tissue inhibitor of metalloproteinases11. In a recent study, periostin was presented as a new biomarker of systemic sclerosis12. Although systemic sclerosis and localized scleroderma share a similar pathomechanism, localized scleroderma is differentiated from systemic sclerosis by clinical characteristics such as the absence of the Raynaud phenomenon and internal organ involvement.
Therefore, we investigated the expression of periostin in the lesional skin tissue of patients with localized scleroderma and compared it to that of age-matched and biopsy site-matched controls. In addition, we evaluated other molecules that have been reported to be associated with the pathogenesis of localized scleroderma, including TGF-β and MMP-1, to determine the role of periostin.
The present study was approved by an internal Institutional Review Board of SMG-SNU Boramae Medical Center (IRB no. 16-2014-118). We performed a retrospective review of electronic medical records from SMG-SNU Boramae Medical Center between January 2011 and February 2014, and we identified 20 patients with localized scleroderma, which was diagnosed by a histopathologic examination. The patients' medical records and specimens, which were stained with hematoxylin and eosin, were reviewed. Patients with early, inflammatory-stage scleroderma (n=1), an unclear diagnosis (n=3), or insufficient data (n=2) were excluded. In addition to the 14 patients with mature, late-stage scleroderma, 14 age-matched (within 10 years) and biopsy site-matched controls were randomly selected from a list of patients who underwent histopathologic examination, but they were diagnosed as having normal skin during the same period.
Formalin-fixed, paraffin-embedded tissue blocks from the patients with localized scleroderma and the controls were retrieved for further analysis. They were sectioned at 4-µm thickness and subjected to the Masson trichrome, periostin, procollagen, α-smooth muscle actin (α-SMA), TGF-β, and MMP-1 stainings. Immunohistochemistry with anti-periostin (Abcam, Cambridge, MA, USA), anti-procollagen 1 (MAB 1912; Chemicon, Temecula, CA, USA), anti-TGF-β (Santa Cruz, Dallas, TX, USA), and anti-MMP-1 (Novus Biologicals, Littleton, CO, USA) were conducted using the BenchMark XT immunostainer (Ventana Medical Systems, Tucson, AZ, USA), according to the manufacturer's protocol. In addition, immunofluorescence analysis was conducted using an antifade medium containing 4′,6-diamidino-2-phenylindole (Molecular Probes/Invitrogen, Carlsbad, CA, USA), periostin (Abcam), α-SMA (Dako, Glostrup, Denmark), and Alexa Fluor® 488- or 594-conjugated donkey antirabbit or antimouse antibodies (Molecular Probes/Invitrogen). Subsequently, we used a confocal microscope (Leica TCS SP8 STED CW; Leica Microsystems, Wetzlar, Germany) to evaluate co-localization of periostin and α-SMA. Images of each section were taken using a digital camera (DP70; Olympus Optical Co., Tokyo, Japan) connected to a light microscope (BX51; Olympus Optical Co.). To visualize collagen deposition, semi-quantitative analysis of sections stained with the Masson trichrome stain was performed by two blinded, independent clinicians, according to the modified criteria of a previous study13. Conflicting assessment results between the two clinicians were reviewed and discussed, and a consensus was reached. Briefly, collagen fiber deposition was evaluated by examining three randomly selected fields of view at ×100 magnification, and it was scored as follows: 0=no collagen fibers, 1=few collagen fibers, 2=moderate amount of collagen fibers, and 3=excessive amount of collagen fibers. Semi-quantitative assessments of periostin and MMP-1 expression were performed by the same clinicians according to the criteria described previously14. The expressions were evaluated as the ratio of the stained area to the entire area per field of view at ×100 magnification, with an average number of three random measurements, and the findings were scored as follows: −, negative staining; +, focal (<5%); 2+, moderate (5%~50%); and 3+, diffuse (>50%) positivity.
Parameters between patients with localized scleroderma and the controls were compared. The chi-square test or Fisher exact test (when at least one cell had <5 counts) was used to analyze categorical variables, and the Wilcoxon signed-rank test was used to analyze continuous variables. Moreover, to investigate correlations among collagen, periostin, and MMP-1, we used the Spearman rank correlation coefficient and data of the patient and controls that were acquired using the aforementioned semi-quantitative analysis method. All statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). A p-value<0.05 was considered statistically significant.
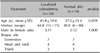
Demographic data for the 14 patients and 14 matched controls are summarized in Table 1. There were no significant differences in age between the two groups. All the controls were sex-matched with the patients, except for one. Patients showed thick, hyalinized, and closely packed collagen bundles in the reticular dermis and subcutaneous layer (Fig. 1A); eccrine glands and adnexal structures were atrophic and trapped within the surrounding collagen bundles. Conversely, the controls had relatively loose, thin collagen bundles with preserved adnexal structures (Fig. 1B). The Masson trichrome staining showed more expansive collagen deposition in the specimens of patients than in those of the controls (Fig. 1C, D). Periostin was widely distributed along the entire dermis and subcutis in patients (Fig. 1E), whereas it was localized along the dermo-epidermal junction in the controls (Fig. 1F). MMP-1 staining in patients showed increased expression in the epidermis and dermis compared to scanty expression in the controls (Fig. 1G, H). Regarding TGF-β and procollagen, there were no differences between the two groups, which means that the patients and controls had scanty expression (Fig. 1I~L). Under the confocal microscope, α-SMA activity was mainly identified at the pericyte and not co-localized with periostin in the patients (Fig. 2).
A semi-quantitative analysis of the collagen bundles showed that patients had a significantly higher proportion of excessive collagen bundle deposition than the controls (57.1% vs. 7.1%, p=0.013; Fig. 3A). Whereas most patients with scleroderma had excessive (n=8, 57.1%) or moderate (n=6, 42.9%) collagen fiber, most of the controls had moderate (n=11, 78.6%) or few (n=2, 14.3%) collagen fibers. Only one control specimen had excessive collagen fibers, and this specimen was obtained from the back, which normally has increased collagen. A semi-quantitative assessment of periostin expression showed a higher proportion of diffuse periostin positivity (42.9% vs. 0%, p=0.016) in patients than in the controls (Fig. 3B). As diffuse MMP-1 expression was lacking in the patient group and control group, we compared the total proportion of moderate expression in the semi-quantitative assessment of MMP-1. The result showed a higher proportion of MMP-1 positivity (71.4% vs. 7.1%, p=0.001) in patients than in the controls (Fig. 3C). In addition, we found a statistically significant correlation between collagen and periostin (rho=0.750, p=0.007) in the patient group, but not in the control group. There were no significant correlations between the other variables in the patient and control groups.
Scleroderma is a chronic connective tissue disease characterized by the excessive accumulation of ECM components, which results in skin fibrosis. Scleroderma is classified as localized scleroderma and systemic sclerosis by association with the Raynaud phenomenon, acrosclerosis, and internal organ involvement. Although there have been many hypotheses, the pathogenesis has not yet been clearly established. Some in vitro studies have shown that localized scleroderma and systemic sclerosis have common features in their pathomechanism such as the pattern of collagen synthesis15161718. Although there are similarities between localized scleroderma and systemic sclerosis, they also have some differences, including autoantibody specificity192021 and the aforementioned clinical findings. Previous studies showing the role of periostin in the pathomechanism were mostly conducted on systemic sclerosis. Therefore, we attempted to investigate whether this association could also be found in localized scleroderma.
When explaining the pathogenesis of scleroderma, various factors have been mentioned in the gene, molecular, and cellular level. Among them, TGF-β is considered a key molecule: TGF-β activates fibroblasts, induces α-SMA expression and myofibroblast differentiation, and achieves diverse effects such as increased ECM deposition using the downstream signaling pathway222324. Similarly, there are many reports on the association between excessive TGF-β activity and pathologic fibrosis in scleroderma92526272829. However, human in vivo studies about TGF-β have shown inconsistent results. Some studies have shown that patients with scleroderma have an increased TGF-β level in their skin and serum compared to a control group3031. In contrast, other studies have failed to show differences in the TGF-β level between patients with scleroderma and controls3233. These conflicting reports may indicate the diverse features of the disease at different stages. Querfeld et al.34 reported that TGF-β activity was mainly involved in the inflammatory phase of scleroderma, not in the sclerotic phase.
In addition to TGF-β, recent in vitro and animal in vivo studies have implicated periostin as a new contributor to the development of skin fibrosis133536. For example, wild-type mice showed faster wound healing than knock-out mice with periostin, because periostin accelerates keratinocyte proliferation and activated dermal fibroblasts3536. Additionally, Yang et al.13 reported that the periostin expression was increased in bleomycin-induced sclerotic skin of wild-type mice compared to that in skin from sham-treated animals. Furthermore, bleomycin was unable to induce scleroderma in knock-out mice with periostin. They demonstrated that periostin was required for α-SMA-positive myofibroblast differentiation and excessive collagen production through the augmentation of TGF-β1 activity. However, there have been insufficient human in vivo data to support these findings. Recently, Yamaguchi et al.12 reported that periostin was markedly increased in the lesional dermis and serum of patients with systemic sclerosis compared to normal controls. Additionally, periostin was co-localized with α-SMA-positive myofibroblasts in lesional dermis, which suggests that periostin is important in the pathogenesis of systemic sclerosis.
Our study's findings demonstrated that periostin was also significantly increased in the skin samples from patients with localized scleroderma compared to those from the controls. Furthermore, substantial periostin expression was observed in the fibrotic dermis with increased collagen production in patients with localized scleroderma, which was consistent with the pattern of systemic sclerosis12. We also found a statistically significant positive correlation between collagen fiber deposition and periostin expression in the patient group.
To investigate the role of periostin in the pathogenesis of localized scleroderma, we performed additional studies that included TGF-β, α-SMA, MMP-1, and procollagen. First, our results failed to show the increased expression of TGF-β in the localized scleroderma skin. This result may be due to the characteristics of study populations that only included patients with mature, late-stage scleroderma.
Second, we assessed the α-SMA expression to identify co-localization of α-SMA-positive myofibroblast and periostin. In patients with localized scleroderma, α-SMA expression was mainly located in the pericyte, so we could not find co-localization of α-SMA myofibroblast and periostin, which was different from that found in a previous report12. This does not mean that there is no association between periostin and myofibroblast in the localized scleroderma. Rather, it suggests that periostin may be secreted from myofibroblasts in the early stage, as reported by Yamaguchi et al.12, and the unrevealed function persists to maintain fibrosis even after the regression of myofibroblasts.
Third, we examined the MMP-1 expression to determine the ECM balance in mature scleroderma, and we found a paradoxical increase in the expression of MMP-1. As previously mentioned, most in vitro data have repetitively shown a decreased expression of MMP-1 in patients' fibroblasts and its protective effect in those with scleroderma5678. Theoretically, it can be expected that MMP-1 expression is decreased in the fibrotic skin of patients with scleroderma, because MMP-1 can degrade ECM components like collagen. However, some conflicting data have also been reported. For example, one study reported that MMP-1 or MMP-3 expression increased in the fibroblasts of patients with early-stage, systemic sclerosis11. Other animal in vitro studies have shown that the synthetic MMP inhibitor had a protective effect on the progression of fibrosis373839. One possible explanation for this paradoxical phenomenon is that MMP may have additional effects on fibrosis other than ECM degradation, e.g., they interact with other signaling pathways such as TGF-β, CTGF, TNF-α, interleukin-1β, and periostin38404142. Secondly, MMP activation may be a result of efforts to achieve homeostasis in a fibrotic state.
Finally, there was no increased procollagen activity in patients with localized scleroderma, which means that there was no active fibrotic process.
Previous studies have shown that periostin plays a role in collagen integrity by attaching to multiple ECM proteins4344. Yamaguchi et al.12 suggested that periostin may be associated with fibrosis by cross-linking collagen in patients with systemic sclerosis. However, based on our findings, we additionally hypothesized that increased periostin in mature stage scleroderma may contribute to the maintenance of fibrosis by making it resistant to the enzyme involved in ECM degradation such as MMP-1. In the early inflammatory phase, TGF-β may be a key molecule that activates downstream cascades, including dermal fibroblast stimulation, myofibroblast differentiation, periostin induction, excessive collagen production, and final skin fibrosis. In the mature sclerotic phase, periostin may maintain the fibrotic state even after the cessation of active fibrotic process by cross-linking collagen fibrils and resistance to the degradation of collagen.
Our study is the first human in vivo study that investigated periostin in patients with localized scleroderma, as only patients with systemic sclerosis have been previously studied. In addition, we studied other molecules that have been considered associated with the pathogenesis of scleroderma so that we could speculate the role of periostin. Another strength of our study is that we only included patients with mature stage scleroderma so we could control the conflicting results according to the different disease stages.
This study has several limitations, including a small number of cases, retrospective study design, and phenomenal findings. Furthermore, we could not obtain peri-lesional skin specimens of patients and the controls for further comparison because of the retrospective study design. Nonetheless, our study's results contribute significant human in vivo data to support previous findings and implicate the pathogenesis of the disease. Further studies are needed to evaluate the serial expression of periostin in accordance with the progression of scleroderma with a larger number of cases for better understanding the role of periostin in scleroderma.
Figures and Tables
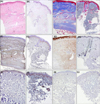 | Fig. 1(A) A representative image of localized scleroderma specimen revealing thick and hyalinized collagen bundles (H&E, ×50). (B) A representative image of control skin without evidence of pathologic findings (H&E, ×50). (C) Excessive collagen bundle deposition in the same scleroderma specimen (Masson trichrome, ×50). (D) Moderate amount of collagen fibers in the same control (Masson trichrome, ×50). (E) Diffuse periostin positivity through the whole dermis in the same scleroderma specimen (anti-periostin antibodies, ×50). (F) Focal positivity of periostin mainly along dermo-epidermal junction in the same control (anti-periostin antibodies, ×50). (G) Moderate matrix metalloproteinase (MMP)-1 positivity in the epidermis and dermis in the same scleroderma specimen (anti-MMP-1 antibodies, ×50). (H) Scanty MMP-1 positivity in the same control (anti-MMP-1 antibodies, ×50). (I) Scanty TGF-β expression in the scleroderma specimen (anti-TGF-β antibodies, ×50). (J) Scanty TGF-β expression in the control (anti-TGF-β antibodies, ×50). (K) Scanty procollagen expression in the scleroderma specimen (anti-procollagen 1 antibodies, ×50). (L) Scanty procollagen expression in the control (anti-procollagen 1 antibodies, ×50). |
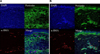 | Fig. 2(A) Scleroderma patient specimen sections are stained with 4′,6-diamidino-2-phenylindole (DAPI), periostin and α-smooth muscle actin (α-SMA). Periostin showed diffuse positivity through the whole dermis. α-SMA expression is mainly located at pericyte and is not colocalized with periostin expression. (B) Control specimen sections are stained with DAPI, periostin and α-SMA. Periostin showed focal positivity along dermo-epidermal junction and perivascular area. α-SMA expression is mainly located at pericyte and is somewhat colocalized with perivascular periostin positivity. |
 | Fig. 3(A) Significantly higher proportion of excessive collagen bundle deposition (57.1% vs. 7.1%, p=0.013). (B) Diffuse periostin expression (42.9% vs. 0%, p=0.016) in the localized scleroderma specimens compared to controls. (C) Moderate matrix metalloproteinase (MMP)-1 expression (71.4% vs. 7.1%, p=0.001) in the localized scleroderma specimens compared to controls. *p<0.05, **p<0.01. |
Table 1
Demographics of patients with localized scleroderma and their controls
ACKNOWLEDGMENT
This study was supported by Grant no. 04-2008-0730 from the Seoul National University Hospital Research Fund.
References
1. Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993; 294:271–278.

2. Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, et al. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 2004; 229:857–868.

3. Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008; 2:9–17.

4. Oku E, Kanaji T, Takata Y, Oshima K, Seki R, Morishige S, et al. Periostin and bone marrow fibrosis. Int J Hematol. 2008; 88:57–63.

5. Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994; 103:359–363.

6. Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006; 126:1761–1769.

7. Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003; 120:542–547.

8. Tomimura S, Ogawa F, Iwata Y, Komura K, Hara T, Muroi E, et al. Autoantibodies against matrix metalloproteinase-1 in patients with localized scleroderma. J Dermatol Sci. 2008; 52:47–54.

9. Hasegawa M, Sato S, Takehara K. Augmented production of transforming growth factor-beta by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Arch Dermatol Res. 2004; 296:89–93.

10. Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford). 2008; 47:Suppl 5. v8–v9.

11. Kuroda K, Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res. 1997; 289:567–572.

12. Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, et al. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013; 168:717–725.

13. Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012; 7:e41994.

14. Park HR, Min SK, Cho HD, Kim KH, Shin HS, Park YE. Expression profiles of p63, p53, survivin, and hTERT in skin tumors. J Cutan Pathol. 2004; 31:544–549.

15. Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin North Am. 1996; 22:647–674.
16. Vuorio T, Mäkelä JK, Kähäri VM, Vuorio E. Coordinated regulation of type I and type III collagen production and mRNA levels of pro alpha 1(I) and pro alpha 2(I) collagen in cultured morphea fibroblasts. Arch Dermatol Res. 1987; 279:154–160.

17. Kähäri VM, Sandberg M, Kalimo H, Vuorio T, Vuorio E. Identification of fibroblasts responsible for increased collagen production in localized scleroderma by in situ hybridization. J Invest Dermatol. 1988; 90:664–670.

18. Krieg T, Braun-Falco O, Perlish JS, Fleischmajer R. Collagen synthesis in generalized morphea. Arch Dermatol Res. 1983; 275:393–396.

19. Sato S, Ihn H, Soma Y, Igarashi A, Tamaki T, Kikuchi K, et al. Antihistone antibodies in patients with localized scleroderma. Arthritis Rheum. 1993; 36:1137–1141.

20. Nagai M, Hasegawa M, Takehara K, Sato S. Novel autoantibody to Cu/Zn superoxide dismutase in patients with localized scleroderma. J Invest Dermatol. 2004; 122:594–601.

21. Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am. 1996; 22:709–735.

22. Takehara K. Hypothesis: pathogenesis of systemic sclerosis. J Rheumatol. 2003; 30:755–759.
23. Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006; 168:499–510.

24. Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts. J Clin Invest. 2004; 113:253–264.

25. Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007; 56:334–344.

26. Wu M, Varga J. In perspective: murine models of scleroderma. Curr Rheumatol Rep. 2008; 10:173–182.

27. Kawakami T, Ihn H, Xu W, Smith E, LeRoy C, Trojanowska M. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J Invest Dermatol. 1998; 110:47–51.

28. Pannu J, Gore-Hyer E, Yamanaka M, Smith EA, Rubinchik S, Dong JY, et al. An increased transforming growth factor beta receptor type I:type II ratio contributes to elevated collagen protein synthesis that is resistant to inhibition via a kinase-deficient transforming growth factor beta receptor type II in scleroderma. Arthritis Rheum. 2004; 50:1566–1577.

29. Pannu J, Gardner H, Shearstone JR, Smith E, Trojanowska M. Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum. 2006; 54:3011–3021.

30. Higley H, Persichitte K, Chu S, Waegell W, Vancheeswaran R, Black C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud's phenomenon. Arthritis Rheum. 1994; 37:278–288.

31. Farrell AM, Dean D, Charnock M, Wojnarowska F. Distribution of transforming growth factor-beta isoforms TGFbeta 1, TGF-beta 2 and TGF-beta 3 and vascular endothelial growth factor in vulvar lichen sclerosus. J Reprod Med. 2001; 46:117–124.
32. Restrepo JF, Guzmán R, Rodríguez G, Iglesias A. Expression of transforming growth factor-beta and platelet-derived growth factor in linear scleroderma. Biomedica. 2003; 23:408–415.

33. Dańczak-Pazdrowska A, Kowalczyk MJ, Szramka-Pawlak B, Gornowicz-Porowska J, Szewczyk A, Silny W, et al. Transforming growth factor-β1 in plaque morphea. Postepy Dermatol Alergol. 2013; 30:337–342.

34. Querfeld C, Eckes B, Huerkamp C, Krieg T, Sollberg S. Expression of TGF-beta 1, -beta 2 and -beta 3 in localized and systemic scleroderma. J Dermatol Sci. 1999; 21:13–22.
35. Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012; 21:331–336.

36. Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011; 6:e18410.

37. Ro Y, Hamada C, Inaba M, Io H, Kaneko K, Tomino Y. Inhibitory effects of matrix metalloproteinase inhibitor ONO-4817 on morphological alterations in chlorhexidine gluconate-induced peritoneal sclerosis rats. Nephrol Dial Transplant. 2007; 22:2838–2848.

38. Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001; 89:201–210.
39. Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001; 193:538–545.

40. Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994; 370:555–557.

41. Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998; 161:3340–3346.
42. Narayanan AS, Page RC, Swanson J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-beta in the presence of other inflammatory mediators. Biochem J. 1989; 260:463–469.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download