Dear Editor:
Primary cutaneous gamma-delta T-cell lymphoma (PCGD-TCL) accounts for less than 1% of primary cutaneous T-cell lymphomas (CTCLs). PCGD-TCL is characterized by a highly aggressive clinical course, while mycosis fungoides (MF) has a relatively favorable prognosis12. PCGD-TCL is recognized for its complexity and heterogeneity3. Here we present a case of PCGD-TCL with Pautrier microabscess.
A 69-year-old female presented with erythematous tumors and plaques on her trunk and extremities. Initially, erythemas and plaques had been noticed at her lower extremities and had spread to her trunk and upper extremities in the past year and a half. Physical examination revealed multiple erythematous tumors and plaques on her trunk and extremities (Fig. 1). Histopathological examination from an erythematous plaque showed focal infiltrates of atypical lymphocytes in the dermis (Fig. 2A, B). Epidermotropic infiltrates of atypical lymphocytes and aggregations of atypical lymphocytes as Pautrier's microabscess in the epidermis were recognized (Fig. 2C). Flow cytometric analysis and immunohistochemistry both showed positive reactions for CD2, CD3 and CD5, a partially positive reaction for CD4, and negative reactions for CD7, CD8, CD20 and CD56 in atypical lymphocytes (Fig. 2D). A positive reaction for CγM1 and negative reactions for T-cell restricted intracellular antigen-1 and granzyme B were demonstrated by immunohistochemistry (Fig. 2E). Atypical lymphocytes in the epidermis were positive for C γM1 and negative for βF1 (Fig. 2F). Positron emission tomography revealed multiple accumulation sites with fludeoxyglucose in the skin and inguinal lymph nodes. Histopathological examination of a lymph node showed medium to large sized atypical lymphocytes. Flow cytometric analysis and immunohistochemistry of the lymph node both showed identical results with the skin in addition to a positive reaction for CγM1 by immunohistochemistry (Fig. 2G). Southern blots showed TCR gene rearrangements by probes of Jγ. A diagnosis of PCGD-TCL was made. Treatments with radiation and cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone (CHOP) had diminished most tumors and plaques, but the tumors recurred. CHOP therapy excluding oncovin due to peripheral neuropathies was effective, and most tumors and plaques have been under control for two years since the start of the initial therapy.
Although PCGD-TCL and MF should be distinct clinical entities in terms of their histogenesis, an accurate diagnosis of PCGD-TCL is sometimes challenging due to the aberrant expression of surface markers in neoplastic cells and technical complexities such as insufficient antibodies that work in paraffin-embedded sections. A previous study of 146 CTCLs showed that 5 PCGD-TCLs and 2 MFs were included in 12 CTCLs with T-cell receptor-γ expression3. Another study of 53 PCGD-TCLs using the same antibody showed that the clinical and histopathological features of 6 cases were similar to those of MF4. Further, PCGD-TCL following MF has also been reported5. Those studies and reports may suggest the heterogeneity of PCGD-TCL. In our case, erythematous plaques were observed in addition to tumors and an epidermotropism as Pautrier microabscess was recognized. Those clinical and histopathological findings may confuse an accurate diagnosis. Detailed analyses of such cases will lead to an accurate diagnosis and the elucidation of the heterogeneity of PCGD-TCL.
Figures and Tables
Fig. 1
(A) Multiple erythematous tumors and plaques on the patient's trunk. The largest tumor was observed at the right lower abdomen. (B) Multiple erythematous tumors on the lower extremities.
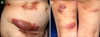
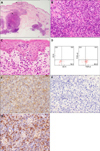
Fig. 2
(A) Histology of a biopsy specimen of an erythematous plaque showing focal infiltrates of atypical lymphocytes in the dermis. The dotted boxes indicate the locations shown at higher magnification in Fig. 1B and 1C (H&E, ×20). (B) Infiltrates of atypical lymphocytes intermingled with histiocytes in the dermis (H&E, ×400). (C) Aggregations of atypical lymphocytes as Pautrier's microabscess in the epidermis (H&E, ×400). (D) Flow cytometry analyses of a biopsy specimen from the skin using a CD45/SSC gating procedure. CD45/SSC gated cells with various surface antigens of CD4, CD8, T-cell receptor (TCR)-αβ, TCR-γδ shown in two dot plots. (E) Immunohistochemistry of a biopsy specimen from the skin showing a positive reaction for CγM1 (×400). (F) Immunohistochemistry of a biopsy specimen from the skin showing a negative reaction for βF1 (×400). (G) Immunohistochemistry from a lymph node showing a positive reaction for CγM1 (×400).
References
1. Toro JR, Liewehr DJ, Pabby N, Sorbara L, Raffeld M, Steinberg SM, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003; 101:3407–3412.

2. Tripodo C, Iannitto E, Florena AM, Pucillo CE, Piccaluga PP, Franco V, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009; 6:707–717.

3. Rodríguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, Tomás IE, Almagro M, Sevilla A, et al. TCR-γ expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013; 37:375–384.

4. Guitart J, Weisenburger DD, Subtil A, Kim E, Wood G, Duvic M, et al. Cutaneous γδ T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012; 36:1656–1665.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download