Abstract
Paraneoplastic pemphigus is a rare, life-threatening autoimmune mucocutaneous blistering disease associated with underlying neoplasia, commonly lymphoproliferative tumors. Herein we report a case of paraneoplastic pemphigus with a unique autoantibody profile associated with a malignant thymoma. A 56-year-old female patient presented with relapsing oral ulcerations accompanied by erythematous papules and patches on her extremities for 2 months. Skin and mucosal biopsies identified interface dermatitis with lichenoid lymphocytic infiltration in the upper dermis. Immunoblotting and enzyme-linked immunosorbent assays revealed that the patient had multiple autoantibodies against desmoglein 1, desmocollin 1, 2, 3, laminin gamma-1, envoplakin, and periplakin. The skin lesions completely healed following thymectomy and systemic corticosteroid therapy, but the oral ulcerations persisted through a follow-up period of over 2 years.
Paraneoplastic pemphigus (PNP) is an autoimmune mucocutaneous disease characterized by an associated underlying neoplasia and the presence of multiple autoantibodies against epithelial proteins1. The clinical and histopathological manifestations of PNP are largely variable2. In some patients, the presentation resembles classic pemphigus and includes painful oral ulcerations and cutaneous blisters as its main features; in others, polymorphous erythematous macules, papules, or plaques are present, similar to those seen in erythema multiforme, lichen planus, or cutaneous graft-vs.-host disease3. The most common and characteristic clinical feature of PNP is severe and intractable mucositis, which may help differentiate the disease from pemphigus vulgaris or oral lichen planus. Genetic predisposition may also be associated with PNP, as specific HLA allele frequencies are increased in PNP patients, depending on ethnicity4. Herein, we report a case of PNP associated with a malignant thymoma in a patient who showed refractory oral ulcerations that persisted even after thymectomy, along with unique immunopathologic features including the presence of anti-desmocollin and anti-laminin gamma-1 antibodies.
A 56-year-old female Korean patient presented with widespread ulcerations involving her oral mucosa, and polymorphous cutaneous lesions over her extremities, for a duration of 2 months (Fig. 1A, B, D). Physical examination showed scattered polygonal erythematous scaly papules and plaques on her distal extremities, particularly the dorsal aspects of her hands and feet. Systemic therapy with prednisolone (up to 15 mg) for 2 months was not effective. Biopsies from the buccal mucosa and the skin over the dorsum of the foot were performed. Histopathology of both lesions demonstrated vacuolar degeneration of the basal cells with lichenoid lymphocytic infiltration in the upper dermis. Several apoptotic keratinocytes were found scattered in the epidermis (Fig. 2). An initial diagnosis of generalized lichen planus was made based on the clinicopathologic findings. Systemic therapy with prednisolone and cyclosporine was initiated, but the oral ulcerations persisted during the 4 months of treatment. The refractory nature of her oral lesions, and the accompanying rapid weight loss led to a suspicion of PNP, and additional immunologic studies were performed. Indirect immunofluorescence (IIF) studies identified IgG deposition on keratinocyte cell surfaces of human skin. IIF performed by using rat bladder demonstrated IgG autoantibodies reactive with the transitional epithelia (Fig. 3A, B). Enzyme-linked immunosorbent assays (ELISA) were positive for desmoglein (Dsg) 1 (index, 34.76; positive, ≥20), desmocollin 1 (optical density [OD], 0.451; cut-off, 0.200), 2 (OD, 0.309; cut-off, 0.070), and 3 (OD, 0.326; cut-off, 0.120), and indeterminate for Dsg3 (index, 13.82; positive, ≥20). Immunoblotting (IB) of normal human epidermal extracts was positive for envoplakin and periplakin, and IB of dermal extracts was positive for laminin gamma-1 (Fig. 3C, D). Computed tomography scanning, which was performed to search for an underlying neoplasm, demonstrated a large anterior mediastinal tumor (Fig. 1E). This was proven to be a malignant thymoma by ultrasound-guided gun biopsy. Total thymectomy, wedge resection of the left upper lobe of the lung, and pericardial resection, were performed. Surgical pathology confirmed the tumor as a thymoma with lung and pericardial extension. Owing to the presence of pleural seeding, she received 45 Gy of adjuvant radiotherapy postoperatively.
Following thymectomy, adjuvant radiotherapy, and systemic therapy with corticosteroids, mycophenolate mofetil, and cyclosporine, skin lesions cleared completely. There was no evidence of tumor recurrence in 19 months postoperatively. Follow-up ELISA for Dsg1 was converted to negative (index, 8.5), and Dsg3 was weakly positive (index, 24.1). However, the oral ulcerations persisted through her follow-up period of more than 2 years.
PNP is an autoimmune disease in which both humoral and cell mediated immune response against epithelial antigens induce skin and mucosal tissue damage. PNP has a diverse spectrum of clinical, pathological, and immunological features. Clinical features include severe, intractable mucositis and polymorphous cutaneous eruptions3, ranging from isolated oral ulcerations to extensive cutaneous blistering. Histopathologic findings depend on the morphology of the clinical lesion, and include intraepidermal acantholysis, interface dermatitis, lichenoid infiltration, and apoptotic keratinocytes5. The mucosal and cutaneous lesions in the present case were characterized by lichenoid infiltration, which led to an initial diagnosis of lichen planus. However, the presence of mucositis intractable to corticosteroids and the histologic findings of apoptotic keratinocytes scattered in the entire epidermis suggested a diagnosis of PNP.
PNP is characterized by the production of multiple autoantibodies against various target agents, including Dsg, bullous pemphigoid antigen 1, desmoplakin, envoplakin, periplakin, and plectin. The gold standard for the diagnosis of PNP is the detection of the characteristic circulating autoantibodies against the 210-kDa envoplakin and the 190-kDa periplakin, by IB6. The present case showed some unique immunologic features. First, all three isoforms of anti-desmocollin antibodies were detected. These antibodies were previously reported in cases of atypical pemphigus, such as pemphigus herpetiformis or pemphigus vegetans, and have also been described in PNP7. They may play a pathogenic role in the non-classical types of pemphigus. Second, antibodies against the 200-kDa laminin gamma-1 were identified. This is the second case of PNP with anti-laminin gamma-1 antibodies8. The role of these antibodies is yet to be described.
Although most patients with PNP exhibit circulating anti-Dsg3 antibodies that contribute to blister formation, there are reports of identification of anti-Dsg1 antibodies alone on ELISA6, as seen in the present case. The pathogenesis of oral lesions in PNP remains unclear, but the presence of autoantibodies against Dsg3 and/or cell-mediated immune responses may play a role in damaging mucosal epithelia9. In general, removal of the underlying neoplasm, especially when the tumors are benign, may lead to clinical improvement and prolonged survival in patients with PNP. However, there are exceptions to this. The persistent oral ulcerations in our patient, even following tumor removal, may have been caused by a persistent T-cell mediated immune response resulting in interface dermatitis. This is supported by the absence of acantholysis on histology and a very low titer of autoantibodies directed against Dsg3.
In conclusion, we report a case of PNP associated with a malignant thymoma in a patient who presented with mucocutaneous lesions with lichenoid infiltrations, unique immunopathologic features, and refractory oral ulcerations that persisted even after thymectomy. Severe oral ulcerations presenting as the sole manifestation in PNP are usually refractory to treatment10. Therefore, careful considerations are needed in such cases.
Figures and Tables
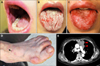 | Fig. 1Clinical findings. The patient initially presented with oral ulcerations (A, B) and erythematous papules on the extremities (D). Oral lesions persist even 3 months following thymectomy (C). A mediastinal tumor was found on chest computed tomography (E, arrowheads), which was later confirmed to be a malignant thymoma. |
 | Fig. 2Histopathologic findings. Skin biopsies of (A) the oral mucosa lesion and (B) the foot lesion. Both of them demonstrate interface dermatitis with diffuse lymphocytic infiltration in the upper dermis. (B) Several necrotic keratinocytes are scattered in the epidermis (H&E; A, B: ×200 magnification). |
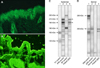 | Fig. 3Immunologic findings. (A) Indirect immunofluorescence demonstrates IgG deposition at the intercellular space of human skin at 1:40 dilution and (B) IgG deposition at the epithelia of rat bladder at 1:10 dilution. (C) Immunoblotting of normal human epidermal extracts is positive for envoplakin (210 kDa) and periplakin (190 kDa; arrows). (D) Immunoblotting of dermal extracts is positive for laminin gamma-1 (200 kDa). BP: bullous pemphigoid, PNP: paraneoplastic pemphigus, PV: pemphigus vulgaris, EBA: epidermolysis bullosa acquisita. |
References
1. Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990; 323:1729–1735.

3. Nguyen VT, Ndoye A, Bassler KD, Shultz LD, Shields MC, Ruben BS, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001; 137:193–206.
4. Liu Q, Bu DF, Li D, Zhu XJ. Genotyping of HLA-I and HLA-II alleles in Chinese patients with paraneoplastic pemphigus. Br J Dermatol. 2008; 158:587–591.

5. Sehgal VN, Srivastava G. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Int J Dermatol. 2009; 48:162–169.

6. Choi Y, Nam KH, Lee JB, Lee JY, Ihm CW, Lee SE, et al. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012; 39:973–981.

7. Ohzono A, Sogame R, Li X, Teye K, Tsuchisaka A, Numata S, et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br J Dermatol. 2015; 173:1447–1452.

8. Oh SJ, Lee SE, Hashimoto T, Kim SC. A case of paraneoplastic pemphigus associated with Castleman disease reacting with multiple autoantigens, including the p200 protein. Br J Dermatol. 2016; 174:930–932.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download