This article has been corrected. See "Corrigendum: The Effect of Micro-Spicule Containing Epidermal Growth Factor on Periocular Wrinkles" in Volume 29 on page 828.
Abstract
Background
Micro-needle patches have been recently used to increase skin permeability, which improves drug delivery, and for cosmetic purposes. However, these patches may often have limited efficacy due to insufficient skin penetration and reduced compliance caused by discomfort.
Objective
We evaluated the efficacy and the safety of soluble micro-spicule containing epidermal growth factor (MS-EGF) for the treatment of periocular wrinkles.
Methods
Twenty healthy volunteers aged 33 to 54 years were enrolled in a randomized, controlled, split-face study. For 4 weeks, a periocular wrinkle was treated daily with either a soluble MS-EGF cream or a cream containing EGF alone. All subjects underwent 8 weeks of follow-up. Efficacy was assessed using an ultrasonic measurement of dermal depth and density, digital skin image analysis, 5-point photonumeric scale for periocular wrinkles and subjective satisfaction.
Results
MS-EGF group showed statistically significant increase of dermal depth and density compared to EGF alone group after 4 and 8 weeks. In addition, there was a marked improvement shown in clinical and 3-dimensional skin image in MS-EGF group. The treatments were well-tolerated; no significant side-effect was noted.
Reducing facial wrinkles has been one of the more popular cosmetic aims, as with people wanting to live healthier, they also want to look younger. Consequently, the use of anti-wrinkle treatments and functional cosmetics is increasing1. For example, anti-wrinkle treatments such as laser therapy, various dermal filler injections and invasive procedures like thread lifting are becoming more widely used23. However, such treatments are usually expensive, they may cause discomfort, and are procedures that require professional intervention.
Skin wrinkles appear due to loss of elasticity caused by rapid degradation of collagen4. These changes are influenced by intrinsic and extrinsic factors that involve mitogenic reactions and signal transduction pathways. Many receptors for epidermal growth factor (EGF), platelet-derived growth factor, interleukin-1, tumor necrosis factor participate in this process5. EGF stimulates and regulates the proliferation of various cell types in vitro6, and for skin cell types, EGF plays an important role in growth and regeneration of keratinocytes and fibroblasts. As such, EGF might be a potential therapeutic and cosmetic agent for damaged skin and injuries including wrinkles and aging7.
The epidermis of skin contains stratum corneum, which consists of keratinocytes that act as “bricks” and intercellular lipids that act as “mortar.” Intracellular lipids act as a barrier against many agents including drugs and various cosmetic agents891011. For these reasons, a topical application of EGF has limited efficacy on treating wrinkles.
Various studies have been conducted to find ways to improve the skin penetration of drugs and cosmetics. For example, micro-needles for transdermal drug delivery have been developed in various forms1213. Micro-needle patches have also been recently used to increase skin permeability, improving drug delivery and for also for various cosmetic purposes14. However this technology often allows for insufficient skin penetration, and there is also low compliance with its use due to discomfort. Accordingly, we developed micro-spicules containing epidermal growth factor (MS-EGF, also called micro-needle EGF) to increase dermal penetration of EGF, and in this study, we investigated the feasibility of MS-EGF as a new technique for drug delivery by assessing its efficacy on wrinkle reduction, along with its safety profile.
MS-EGF is a 0.25-µm pyramidal shaped material composed of hyaluronic acid. This micro-spicule contains EGF and is readily soluble in water. MS-EGF cream is MS-EGF mixed with a general hydrophobic cosmetic paste (Fig. 1A).
We performed an ex vivo skin penetration test using pig skin (Medikinetics, Pyeongtaek, Korea) to investigate the penetration ability of MS-EGF in epidermis. The pig skin samples were divided into three groups: untreated, MS-EGF and EGF alone group. For the MS-EGF group, 100 mg of MS-EGF was scrubbed for 20 seconds and then distilled water was added to dissolve the remaining micro-spicules, followed by additional rubbing for 20 seconds. For the EGF alone group, 100 mg of EGF was applied for 40 seconds. All the sample groups were observed at room temperature for 10 minutes and then stored at 4℃ for 12 hours. Tissue samples were fixed with 10% formaldehyde, embedded in paraffin, and cut into sections. The sections were deparaffinized and processed with Bond™ polymer refine detection kit (Leica Microsystems, Wetzlar, Germany). The primary EGF antibody (Santa Cruz Biotechnology, Dallas, TX, USA) was diluted to 1:50 and the sections were incubated with secondary antibody at room temperature for 10 minutes. The sections were incubated with diaminobenzidine tetrachloride solution at room temperature for 5 minutes and counterstained with 0.1% Mayer's hematoxylin.
Twenty healthy volunteers were enrolled in this randomized, controlled, left side-right side split-face test. The participants were over 30 years old with mild to moderate periocular wrinkles. All the volunteers were exposed to the same external environment. Demographic data such as age, gender, and past medical history was collected prior to enrollment. Volunteers receiving therapeutic interventions such as botulinum toxin or fillers and those with concomitant cutaneous diseases such as acne and scar on periocular region were excluded from the study.
Participants were asked to apply either MS-EGF cream or EGF cream on each periocular wrinkle on a daily basis for 4 weeks. MS-EGF participants were told to apply the cream for 20 seconds, add distilled water to dissolve the remaining micro-spicules for the purpose of preventing irritation, and then rubbing again for 20 seconds. EGF alone participants were told to apply the cream for 40 seconds with the same intensity. This study was approved by the Institutional Review Board of Chungnam National University Hospital (CNUH 2015-05-013). All of the subjects provided written informed consents before participating in the study.
The participants were assessed at the beginning of the treatment and at 1, 2, 4, and 8 weeks of treatment regimen. A-One Pro digital skin image analysis equipment (Bomtech Electronics Co., Seoul, Korea) for objective evaluation of wrinkle degree and 3-dimensional (3D) skin image of periocular wrinkles was used during each visit. Dermal density and dermal depth were measured by ultrasonic equipment, Ultrascan UC22 (Courage Khazaka Electronics, Cologne, Germany). The severity of periocular wrinkles was assessed according to a 5-point photonumeric scale. The scale ratings are: 0 for no wrinkles, 1 for very fine wrinkles, 2 for fine wrinkles, 3 for moderate wrinkles, and 4 for severe wrinkles15. At the end of the study, the patients documented their degree of satisfaction as very satisfied, satisfied, slightly satisfied, or unsatisfied. Patients were also asked to report any side effects during each visit.
Statistical analyses were performed using SPSS ver. 15.0 (SSPS Inc., Chicago, IL, USA). The Mann-Whitney U-test was used for post hoc analysis. To compare the categorical data, the chi-square test or the Fisher exact test was performed. Correlations were performed using the Spearman correlation analysis. p-values<0.05 were considered to be statistically significant.
In a preliminary study using pig skin, stratum corneum was effectively removed and EGF in the epidermis was uniformly distributed in the MS-EGF group. EGF alone group also showed a weak presence of EGF in the epidermis; however, there was no significant difference compared to the untreated group (Fig. 1B).
A randomized, controlled, left-right face spilt test was performed afterwards to test the efficacy of MS-EGF on periocular wrinkle. A total of 20 healthy volunteers (16 females, 4 males) with an average age of 45.2 years (range, 33~54 years) were enrolled in this study. There were no significant differences in the degree of baseline wrinkles between each group. After daily applications for each group for 4 weeks on each periocular wrinkle, the MS-EGF grouped showed a marked improvement at 8 weeks after topical application, shown with skin ultrasound analysis (Fig. 2A). The mean dermal density of both groups at baseline was similar (6.25%±1.73% in MS-EGF vs. 6.38%±1.55% in EGF alone) and this value was set as a standard of the dermal density ratio changes. In the MS-EGF group, dermal density ratio was significantly improved at 4 and 8 weeks after treatment, respectively (p<.0.01, p<.0.001). In the EGF alone group, there was a slight increase in dermal density, but the difference was not statistically significant. The MS-EGF group also demonstrated a significant increase in dermal density after 4 and 8 weeks compared to EGF alone group, respectively (p<.0.05, p<.0.01; Fig. 2B).
Results for mean dermal depth were similar to those for dermal density. Although mean dermal depths of both groups at baseline were similar (1.75±0.30 mm in MS-EGF vs. 1.80±0.46 mm in EGF alone), MS-EGF group showed a significantly increase in dermal depth after 8 weeks compared to the EGF alone group (p<.0.05; Fig. 2C). From the above observations, there was a 30.1% increase in dermal density and a 19.5% increase in dermal depth from baseline in the MS-EGF group.
Clinical digital photographs and 3D image of periocular wrinkles by A-One Pro image analysis were used for clinical comparisons. Although both groups showed favorable results after 8 weeks compared to the baseline, the clinical photograph and 3D skin image improved significantly more with MS-EGF treatment than that for EGF alone treatment (Fig. 3A, B). Wrinkle severity score was evaluated automatically with A-One Pro, using a 5-point photonumeric scale. In the A-One Pro measurements, wrinkle scores decreased in both groups; however, the MS-EGF group showed significant improvement in wrinkle score compared to EGF alone group after 8 weeks (p<.0.05) (Fig. 3C). In the 5-point photonumeric scale, there was no significant difference in the grades between the two groups; however, the MS-EGF group showed a greater change in mean grade using this scale (2.5~2.0 in MS-EGF vs. 2.4~2.2 in EGF alone group; Fig. 3D).
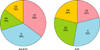
Subjective improvement was also assessed by all participants upon completing the study. Among the participants who received MS-EGF treatment, 70% were “very satisfied” or “satisfied” after the treatment, whereas only 15% were “dissatisfied.” For the EGF alone group, 50% were “very satisfied” or “satisfied” after treatment whereas 20% were “dissatisfied” (Fig. 4). The treatments were well-tolerated by most patients and there were no noticeable adverse events such as inflammation, desquamation and pigmentation in either of the treated areas. Six participants in MS-EGF experienced transient erythema and a prickling sensation immediately after the application, which resolved within a few minutes without special management.
Wrinkles are one of the most common morphological features of skin aging16. Interest in anti-aging is growing every day as people pay more attention to beauty and their looks. For these reasons, the interest in functional anti-aging cosmetics has increased for most age groups117, and many cosmetic products targeted at reducing signs of aging have been developed. Despite various approaches in developing cosmetics, these products have limited skin penetration due to the skin barrier. Therefore, various molecular, chemical, and morphological studies have been performed on increasing the skin penetration of various topical agents18192021.
Micro-needle technology offers an efficient and minimally invasive drug delivery compared to conventional transdermal patches and intradermal injections22. With the advancement of micro-units manufacturing technology, micro-needles have been developed by academic laboratories and pharmaceutical companies, and are currently being used to enhance transdermal delivery of various molecules2324. Micro-needles increase the patients' compliance as they may have a regular needle phobia so that patients can apply the drug by themselves25. Micro-needles in form of patches are band-aid like materials with embedded micro-needles fabricated in arrays and can be in four versions such as hollow, solid, coated and polymer forms. However, there are some limitations for using these patches. First, skin pore closure needs to be additionally investigated after micro-needle patches application as it relates to the risk of infections. Second, it is difficult to control the drug amount in micro-needle patches26. To overcome these challenges, a new drug delivery technique with higher efficacy and safety is required.
This was the first study to investigate a soluble micro-spicule type topical agent for anti-wrinkle improvement. In experiments using pig skin, micro-spicule cream showed higher skin penetration ability than a typical cream, containing the same ingredients. This method allows delivery of various agents, but with a topical application. As such, it was possible to evaluate the anti-wrinkle efficacy and safety of soluble MS-EGF, delivering EGF beyond the external skin barrier.
In the randomized and controlled face spilt trial of 20 healthy volunteers who had MS-EGF and EGF in a cream form, periocular wrinkles were clinically improved in both groups after 8 weeks. However, there were significant differences in dermal density, dermal depth, and wrinkle severity after 4 and 8 weeks between the two treatment forms. Anti-wrinkle improvement with MS-EGF was more significant and superior to the EGF alone treatment. In the study, the skin penetration ability for EGF was also larger with MS-EGF. This implied the increased anti-wrinkle efficacy of MS-EGF was due to superior skin penetration ability from the micro-spicules. Most participants were more satisfied when using MS-EGF and consider that micro-spicules might find more applications and be more favored, despite the scrubbing of particles, and regardless of pain during the application. Although there was a temporary erythema and mild pain on MS-EGF lesion when rubbing strongly, it led to a better clinical outcome.
There has been one report on clinical improvements of periocular wrinkle using micro-needle patch including EGF. It showed the positive effects for the micro-needle EGF patch on physician-rated wrinkle scores, patient satisfaction levels, and corneometer results. However, there were no statistical differences compared with the control group, hyaluronic acid-based and without EGF micro-needle patches. This implied that the anti-wrinkle effects of the micro-needle patch may had been solely be due to the HA rather than EGF in the patches27. To further delineate the advantage of MS-EGF, in the future, additional studies need to compare the efficacy of EGF micro-needle patches with MS-EGF cream technology for wrinkle reduction.
In conclusion, the results of this study identified a favorable clinical benefit for MS-EGF in treating periocular wrinkles along with good tolerability and higher satisfaction from the volunteers that used it. Furthermore, the soluble property of micro-spicules is noteworthy for a new type of cosmetics delivery system and has more development potential in various other applications.
Figures and Tables
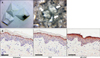 | Fig. 1Appearance and penetration assay of micro-spicule containing epidermal growth factor (MS-EGF). (A) A 0.25-µm-sized pyramidal shaped hollow spicule made of hyaluronic acid (left) and MS-EGF mixed with general hydrophobic cosmetic paste (right) (arrows: MS-EGF component, ×200). (B) Histological finding showing epidermal growth factor (EGF) expression in the skin after application of MS-EGF (anti-EGF stain, Scale bar=50 µm). |
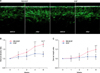 | Fig. 2Measurement of dermal depth and density using skin ultrasound. (A) One ultrasound image was taken at baseline (left) and the other was taken 8 weeks after (right) for the micro-spicule containing epidermal growth factor (MS-EGF) and epidermal growth factor (EGF) alone group. Change of dermal density (B) and depth (C) ratio from baselines. *p<0.05, **p<0.01, ***p<0.001 vs. baseline; †p<0.05, ††p<0.01 vs. EGF. |
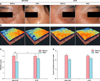 | Fig. 3Clinical outcome of periocular wrinkle using digital skin image and wrinkle score. (A) Photographs showing clinical improvements of periocular wrinkles. One photograph was taken at baseline (left) and the other was taken 8 weeks after (right). (B) Three-dimensional analysis images of baseline (left) and 8 weeks after (right). Red color indicate upper high and blue color indicates lower high. (C) Wrinkle score using skin image analysis. (D) Wrinkle score using 5-point photonumeric scale. MS-EGF: micro-spicule containing epidermal growth factor, EGF: epidermal growth factor. *p<0.05, ***p<0.001. |
ACKNOWLEDGMENT
This study was supported by a grant of the R&D for Regional Industry, Ministry of Trade, Industry and Energy, Republic of Korea (Grant no. A009000256). Paean Biotechnology Inc. (Daejeon, Korea) provided the research funding, but had no participation in the data analysis, or the process of manuscript preparation and submission.
References
1. Rinaldi A. Healing beauty? More biotechnology cosmetic products that claim drug-like properties reach the market. EMBO Rep. 2008; 9:1073–1077.
2. Ooe M, Seki T, Miura T, Takada A. Comparative evaluation of wrinkle treatments. Aesthetic Plast Surg. 2013; 37:424–433.

3. Narayan RJ. Transdermal delivery of insulin via microneedles. J Biomed Nanotechnol. 2014; 10:2244–2260.

4. Farage MA, Miller KW, Elsner P, Maibach HI. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008; 20:195–200.

5. Chauhan P, Shakya M. Modeling signaling pathways leading to wrinkle formation: identification of the skin aging target. Indian J Dermatol Venereol Leprol. 2009; 75:463–468.

6. Anchan RM, Reh TA, Angello J, Balliet A, Walker M. EGF and TGF-alpha stimulate retinal neuroepithelial cell proliferation in vitro. Neuron. 1991; 6:923–936.

7. Schlessinger J, Schreiber AB, Levi A, Lax I, Libermann T, Yarden Y. Regulation of cell proliferation by epidermal growth factor. CRC Crit Rev Biochem. 1983; 14:93–111.

8. Elias PM, Friend DS. The permeability barrier in mammalian epidermis. J Cell Biol. 1975; 65:180–191.

9. Elias PM, Friend DS, McNutt NS. Epidermal permeability barrier: transformation of lamellar granule-disks into intercellular sheets by a membrane fusion process. J Invest Dermatol. 1987; 88:459–460.

10. An JJ, Eum WS, Kwon HS, Koh JS, Lee SY, Baek JH, et al. Protective effects of skin permeable epidermal and fibroblast growth factor against ultraviolet-induced skin damage and human skin wrinkles. J Cosmet Dermatol. 2013; 12:287–295.

11. Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983; 80:Suppl. 44s–49s.

13. van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012; 161:645–655.

14. Doraiswamy A, Ovsianikov A, Gittard SD, Monteiro-Riviere NA, Crombez R, Montalvo E, et al. Fabrication of microneedles using two photon polymerization for transdermal delivery of nanomaterials. J Nanosci Nanotechnol. 2010; 10:6305–6312.

15. Carruthers A, Carruthers J, Hardas B, Kaur M, Goertelmeyer R, Jones D, et al. A validated grading scale for crow's feet. Dermatol Surg. 2008; 34:Suppl 2. S173–S178.

16. Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996; 135:867–875.

17. Binstock RH. Anti-aging medicine and research: a realm of conflict and profound societal implications. J Gerontol A Biol Sci Med Sci. 2004; 59:B523–B533.
18. Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006; 123-126:369–385.

19. Schramm J, Mitragotri S. Transdermal drug delivery by jet injectors: energetics of jet formation and penetration. Pharm Res. 2002; 19:1673–1679.
20. Tokudome Y, Nakamura K, Itaya Y, Hashimoto F. Enhancement of skin penetration of hydrophilic and lipophilic compounds by pH-sensitive liposomes. J Pharm Pharm Sci. 2015; 18:249–257.

21. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010; 1:109–131.

22. Cai B, Xia W, Bredenberg S, Li H, Engqvist H. Bioceramic microneedles with flexible and self-swelling substrate. Eur J Pharm Biopharm. 2015; 94:404–410.

23. Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010; 99:4228–4238.

24. Donnelly RF, Moffatt K, Alkilani AZ, Vicente-Pérez EM, Barry J, McCrudden MT, et al. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm Res. 2014; 31:1989–1999.

25. Kaur M, Ita KB, Popova IE, Parikh SJ, Bair DA. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur J Pharm Biopharm. 2014; 86:284–291.

26. Ita K. Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics. 2015; 7:90–105.

27. Park J, Seo J, Shin JU, Jeong DH, Kim JD, Lee KH. Efficacy of biodegradable microneedle patches on periorbital wrinkles. Korean J Dermatol. 2014; 52:597–607.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download