Abstract
Background
Behçet disease (BD) is a relapsing inflammatory disease with increased production of inflammatory cytokines in peripheral blood mononuclear cells (PBMCs); however, the underlying molecular mechanisms are not well known.
Objective
To analyze whether the differential expression of transcription factors is involved in the increased tumor necrosis factor (TNF)-α and interleukin (IL)-6 production by PBMCs of BD patients compared to healthy controls (HCs).
Methods
Expression of transcription factors was examined by real-time reverse transcriptase-polymerase chain reaction and western blotting. Cytokine production by CD11b+ cells transfected with siRNAs against transcription factors was measured by enzyme-linked immunosorbent assay.
Results
In the absence of lipopolysaccharide stimulation, the transcript level of CCAAT-enhancer-binding proteins (C/EBP) β was increased in PBMCs from patients with active BD compared to that in PBMCs from patients with stable BD. The C/EBPδ transcript level was higher in PBMCs from patients with active BD than in those from HCs. The activating transcription factor 3 (ATF3) transcript level was increased in PBMCs from patients with stable BD compared to that in PBMCs from HCs. siRNAs targeting C/EBPβ and C/EBPδ significantly reduced the production of IL-6 and TNF-α in lipopolysaccharide-stimulated CD11b+ cells from patients with BD as well as from HCs.
Behçet disease (BD) is a recurrent inflammatory disease and various genetic, microbial, and immunological factors are believed to be involved in the development of BD1. Abnormal immunological features have been reported in BD such as dysregulation of proinflammatory cytokine production and immunoregulatory cell-surface molecule expression in peripheral blood mononuclear cells (PBMCs) in basal state and/or in response to stimulation with phorbol-12-myristate-13-acetate or lipopolysaccharide (LPS)234. However, the underlying mechanisms of these abnormal immunological features have not been fully elucidated.
Expression of tumor necrosis factor (TNF)-α and interleukin (IL)-6 is under the control of various transcription factors, including CCAAT/enhancer-binding proteins (C/EBPs) and activating transcription factor 3 (ATF3)5. C/EBPβ and C/EBPδ have been demonstrated to induce IL-6 expression through binding to and activating the IL-6 promoter6. ATF3 is induced by toll-like receptor (TLR) stimulation7, and ATF3 deficiency leads to increased production of IL-6 and TNF-α5. The question as to whether these transcription factors play a role in the differential production of IL-6 and TNF-α in patients with BD remains unanswered though.
In this study, we analyzed the expression of transcription factors in PBMCs of BD patients and investigated their roles in the production of TNF-α and IL-6.
Patients with BD and healthy volunteers were recruited at the Ajou University Hospital. BD was defined according to the International Study Group and Japanese criteria for BD. Table 1 shows the demographics and clinical characteristics of the subjects, and Table 2 shows the medications used by patients when the blood samples were collected. All subjects provided informed consent for the study, which was approved by the Ajou University Hospital Institutional Review Board (AJIRB-GEN-GEN-10-119).
PBMCs were separated using Ficoll-Paque Plus (StemCell Technologies, Vancouver, BC, Canada) and CD11b+ cells were isolated using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of CD11b+ cells was analyzed by flow cytometry. The cells (3×106/ml) were treated with 10 ng/ml LPS (Sigma-Aldrich, St Louis, MO, USA) for 3 hours in RPMI1640 medium (Invitrogen, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Invitrogen), penicillin (100 U/ml, Invitrogen), and streptomycin (100 µg/ml, Invitrogen).
LPS level in the sera of study subjects was determined using the ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ, USA) according to the manufacturer's instructions.
Total RNA was extracted with TRIzol (Invitrogen). Real-time reverse transcription-polymerase chain reaction was performed using specific primers for C/EBP-β (5′-GGCCGGTTTCGAAGTTGATG-3′, 5′-AGTTACACGTGGGTTGCGTCAG-3′), C/EBP-δ (5′-CATCGACTTCAGCGCCTACATC-3′, 5′-AAGAGCTCGTCGTGGCACAG-3′), and ATF3 (5′-AAGAGGCGACGAGAAAGAAA-3′, 5′-TGGAGTCCTCCCATTCTGAG-3′). Actin was used for normalization. Polymerase chain reaction was run on an ABI PRISM 7000 Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA).
The concentration of IL-6 and TNF-α in culture media was analyzed using enzyme-linked immunosorbent assay kits (eBioscience, San Diego, CA, USA). The expression of transcription factors in the total cell lysate or nuclear lysate was analyzed by western blot using antibodies against C/EBP-β (Abcam, Cambridge, UK), C/EBP-δ (Abcam), ATF3 (Sigma), histone H3 (Cell Signaling, Danvers, MA, USA), and actin (Bethyl Laboratories, Montgomery, TX, USA), with enhanced chemiluminescence (GE Healthcare Life Sciences, Uppsala, Sweden).
To inhibit the expression of C/EBP-β, C/EBP-δ, and ATF3, siRNAs were purchased from Integrated DNA Technologies (Coralville, IA, USA). THP-1 cells (2.5×104) were transfected with 22.5 nM control oligonucleotide (con siR), siRNA against C/EBPβ, C/EBPδ, or ATF3 or the combination of equal amount of siRNAs against C/EBPβ and C/EBPδ together with 2.5 nM fluorescein isothiocyanate-conjugated control siRNA (FITC-siRNA) (Bioneer, Daejeon, Korea) using Lipofectamine 2000. After 24 hours, the efficiency of transfection was determined using flow cytometry and the expression of each transcription factor was assessed by Western blot. CD11b+ cells (5×105) were similarly transfected with 20 nM siRNA.
Although PBMCs from patients with BD showed differential responses to LPS stimulation, the relevance of in vitro LPS stimulation with BD is not clear. We first determined the LPS concentration in the sera of patients with BD (Fig. 1). Compared to that in healthy controls (HC), LPS concentration was significantly increased in patients with BD (p<0.005). Although the association of infectious agents (herpes virus and gram-positive bacteria) with BD pathogenesis has been reported, our results suggest a possible role for LPS in the pathogenesis of BD. Increased LPS concentration in serum is observed in community-acquired pneumonia usually caused by viruses or gram-positive bacteria, and is reported to contribute to the pathogenesis of pneumonia via NOX2 activation8.
Given that transcription factors such as C/EBPβ, C/EBPδ, and ATF3 are induced 3 hours post-LPS stimulation and regulate the transcription of IL-6 and TNF-α5, we analyzed the mRNA levels of these transcription factors in PBMCs from BD patients (Fig. 2). C/EBPβ mRNA levels were approximately 2-fold higher in unstimulated PBMCs from patients with active BD compared to those in unstimulated PBMCs from patients with stable BD (p<.0.01); however, this increase was not observed following LPS stimulation. C/EBPδ mRNA levels in PBMCs from patients with active BD were significantly higher than those in PBMCs from HCs both in the absence (p<.0.005) and presence of LPS stimulation (p<.0.05). In contrast, ATF3 mRNA expression was increased in PBMCs from patients with stable BD compared to that in the PBMCs from HCs, and was further increased by LPS stimulation (p<.0.05). mRNA levels of these transcription factors did not show significant correlation with HLA-B51 genotype, ocular symptoms, or erythema nodosum (data not shown).
Subsequently, we assessed the protein levels of C/EBPβ, C/EBPδ, and ATF3 in PBMCs by western blotting (Fig. 3). C/EBPβ mRNA can be translated into 3 isoforms (LAP*, LAP, and LIP) using 3 different initiation sites on a single mRNA9. LAP and LIP were detected, but LAP* was not detected in PBMCs from any subject. Unlike mRNA levels, prominent differences in protein levels of C/EBPβ were not observed probably due to the multiple mechanisms controlling C/EBPβ protein levels, including protein stability (half-life of LAP and LIP is approximately 2 hours and 8 hours, respectively). Although not statistically significant, the average ratios of LAP (which transactivates the IL-6 promoter) to LIP (which inhibits LAP activity) were slightly higher in PBMCs from both stable and active BD patients than that of HCs in the presence of LPS. Concordant to mRNA levels, the average protein level of C/EBPδ, a positive regulator of IL-6, tended to increase in PBMCs of active BD patients compared with that in the PBMCs of HCs and stable BD patients. On the other hand, the average nuclear levels of ATF3, a negative regulator of IL-6, did not show significant difference between study groups. Taken together, differential mRNA expression of C/EBPβ, C/EBPδ, and ATF3 was observed in PBMCs of BD patients.
We then evaluated the relevance of differential mRNA expression of C/EBPβ, C/EBPδ, and ATF3 to the increased production of TNF-α and IL-6 in CD11b+ cells of active BD using siRNA. First, we confirmed the successful knockdown of these transcription factors in THP-1 cells transfected with siRNA against each gene, comparing to protein levels observed in non-transfected cells or cells transfected with an unrelated, control siRNA (Fig. 4A). We next transfected CD11b+ cells with siRNAs targeting ATF3 or C/EBPβ alone or for both C/EBPβ and C/EBPδ. However, we could not include the transfection condition of siRNA targeting C/EBPδ alone due to the limited number of CD11b+ cells from each subject. After 24 hours, we transferred cells into fresh media with or without LPS, and 3 hours later assessed the amount of TNF-α and IL-6 in the media (Fig. 4B, C). In PBMCs of stable BD, LPS-induced production of TNF-α and IL-6 was significantly suppressed through transfection of siRNA targeting C/EBPβ alone or C/EBPβ in combination with C/EBPδ, using an equal amount of siRNA mixture for each condition (p<.0.05). Similarly, significant suppression of cytokine production by the transfection of siRNA targeting C/EBPβ alone or C/EBPβ in combination with C/EBPδ was observed in cells of patients with active BD, although modulation of TNF-α by siRNA targeting C/EBPβ alone was not statistically significant. Taken together, these results demonstrate that C/EBPβ and C/EBPδ contribute to the production of TNF-α and IL-6 in PBMCs from patients with BD.
In this study, we evaluated the involvement of transcription factors in the differential production of TNF-α and IL-6 in patients with BD. We showed differential expression of C/EBPβ, C/EBPδ, and ATF3; mRNA of C/EBPβ and C/EBPδ but not ATF3 was higher in PBMCs of active BD, whereas ATF3 mRNA was upregulated in PBMCs of stable BD. Further, a critical role for C/EBPβ and C/EBPδ in the production of TNF-α and IL-6 in CD11b+ cells of BD was demonstrated (Fig. 5).
Currently, the regulation of expression of C/EBP transcription factors in patients with BD has not been reported. However, the association between these transcription factors and autoimmune diseases was previously observed in multiple sclerosis, wherein, C/EBPδ deficiency led to decreased clinical severity in a mouse model of this disease. This was associated with reduced ratios of Th17 to regulatory T cells10. The significance of C/EBPβ in multiple sclerosis was also reported. T cells specific to myelin basic protein, an autoantigen of multiple sclerosis, induced microglial expression of IL-1β, IL-1α, TNF-α, and IL-6, dependent on very late antigen-4-mediated C/EBPβ activation in microglial cells11. Additionally, C/EBPβ involvement in arthritis was reported12. Our results propose that deregulation of transcription factor expression may be one of the underlying mechanisms for abnormal cytokine expression in BD.
Although we did not examine the mechanism of differential expression of C/EBPβ, C/EBPδ, and ATF3 in patients with BD, compared to that in the HCs, our data clearly showed a difference in mRNA levels indicating that transcriptional induction and/or mRNA stability of these transcription factors might be enhanced in patients with BD. Transcription of C/EBPβ and C/EBPδ is increased through MyD88 and an IL-1R-associated kinase 4-dependent signaling pathway6. Additionally, ATF3 is induced by various stimuli, such as TLR agonists (via the nuclear factor-κB pathway) and TNF-α or H2O2 (via the p38 pathway)13. On the other hand, ATF3 mRNA half-life varies from 1 hour in a basal state to 8 hours in stressed states such as amino acid depletion or endoplasmic reticulum stress14. Thus, it is plausible that differential mRNA levels of C/EBPβ, C/EBPδ, and ATF3 in PBMCs from patients with BD might result from differences in signaling pathways regulating the transcription or stability of these mRNAs. Further studies are required to dissect the mechanisms leading to upregulation of C/EBPβ, C/EBPδ, and ATF3 mRNA levels in patients with BD.
In conclusion, our results demonstrated differential expression of C/EBPβ, C/EBPδ, and ATF3 in PBMCs from patients with BD and suggested that these molecules played a regulatory role in the production of TNF-α and IL-6. Further studies regarding the mechanisms underlying differential expression of these transcription factors in PBMCs from patients with BD is warranted to elucidate the pathogenesis of BD.
Figures and Tables
Fig. 1
Increased lipopolysaccharide (LPS) concentration in sera of patients with Behçet disease (BD). LPS concentration was analyzed in the sera of five healthy controls (HCs) and 10 patients with BD (BD), using a Chromogenic LAL Endotoxin Assay Kit. The Mann-Whitney test was conducted. ***p<0.005.

Fig. 2
Differential mRNA expression of CCAAT-enhancer-binding proteins (C/EBP) β (A), C/EBPδ (B), and activating transcription factor 3 (ATF3) (C) in peripheral blood mononuclear cells (PBMCs) from Behçet disease (BD) patients. PBMCs isolated from 5 healthy controls (HCs), 4 to 6 stable BD patients (St) and 6 to 7 active BD patients (Ac) were cultured with or without lipopolysaccharide (LPS) for 3 hours. mRNA levels of C/EBPβ, C/EBPδ, and ATF3 were analyzed by real-time reverse transcription-polymerase chain reaction. Fold over HC(–): Relative mRNA level versus the average mRNA level in HC without LPS stimulation. Each symbol represents a single subject. Bars represent the mean of each group. The Kruskal-Wallis test with Dunn's procedure was conducted. *p <0.05, **p<0.01, ***p<0.005.

Fig. 3
Protein levels of CCAAT-enhancer-binding proteins (C/EBPβ), C/EBPδ, and activating transcription factor 3 (ATF3) in peripheral blood mononuclear cells (PBMCs) from Behçet disease (BD) patients. PBMCs isolated from healthy controls (HCs), stable BD patients (St), or active BD patients (Ac) were cultured with or without lipopolysaccharide (LPS) for 3 hours. Cell lysates were subjected to western blotting. Representative Western blots of nuclear lysates of 4 or 5 independent experiments (A). Relative band intensity to the indicated protein was compared between groups (B). Each symbol represents a subject and the bars represent the mean.

Fig. 4
Suppressive effect of siRNA targeting CCAAT-enhancer-binding proteins (C/EBP) β and C/EBPδ on the production of tumor necrosis factor (TNF)-α and interleukin (IL)-6. (A) Knockdown of the indicated transcription factors using specific siRNA. THP-1 cells were transfected with control siRNA (control) or siRNA specific for C/EBPβ, C/EBPδ, or ATF3. After 24 hours of transfection, protein levels were determined by Western blotting. un t/f: untransfected. (B, C) CD11b+ cells isolated from 5 healthy controls (HCs), 9 stable Behçet disease (BD) patients (St), and 10 active BD patients (Ac) were transfected with the indicated siRNA. After 24 hours, culture media was replaced with fresh media with or without lipopolysaccharide (LPS) (10 ng/ml). After 3 hours, the concentration of TNF-α and IL-6 in the media was measured. Relative production is the ratio of cytokine concentration in each culture condition relative to the average cytokine concentration in the control siRNA (con)-transfected culture without LPS stimulation. Each symbol represents a subject and the bars represent the mean of each group. Con, siRNA for C/EBPβ (siβ), siRNA for ATF3 (siATF3), a combination of siβ and C/EBPδ (siβ+δ). The Mann-Whitney test was conducted. *p<0.05, **p<0.01. un t/f: untransfection.

Fig. 5
Graphical summary. LPS: lipopolysccharide, ATF3: activating transcription factor 3, C/EBPβ LAP: CCAAT-enhancerbinding proteins β liver-enriched transcriptional-activator protein, HC: healthy controls, BD: Behcet disease, IL-6: interleukin-6, TNF-α: tumor necrosis factor-α.
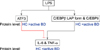
Table 1
Characteristics of healthy controls and patients with BD

Table 2
Drugs taken by patients at the time of sampling

ACKNOWLEDGMENT
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (grant number A101936).
References
1. Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behçet's disease. Autoimmun Rev. 2012; 11:687–698.

2. Sim JH, Park MJ, Park S, Lee ES. Altered expression of costimulatory molecules in Behçet's disease according to clinical activity. Br J Dermatol. 2011; 164:1285–1291.

3. Mege JL, Dilsen N, Sanguedolce V, Gul A, Bongrand P, Roux H, et al. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behçet's disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993; 20:1544–1549.
4. Akman A, Sallakci N, Coskun M, Bacanli A, Yavuzer U, Alpsoy E, et al. TNF-alpha gene 1031 T/C polymorphism in Turkish patients with Behçet's disease. Br J Dermatol. 2006; 155:350–356.
5. Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009; 9:692–703.

6. Lu YC, Kim I, Lye E, Shen F, Suzuki N, Suzuki S, et al. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J Immunol. 2009; 182:7212–7221.

7. Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007; 179:3622–3630.

8. Cangemi R, Pignatelli P, Carnevale R, Bartimoccia S, Nocella C, Falcone M, et al. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J Infect. 2016; 73:107–114.

9. Calkhoven CF, Müller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000; 14:1920–1932.
10. Tsai VW, Mohammad MG, Tolhurst O, Breit SN, Sawchenko PE, Brown DA. CCAAT/enhancer binding protein-δ expression by dendritic cells regulates CNS autoimmune inflammatory disease. J Neurosci. 2011; 31:17612–17621.

11. Dasgupta S, Jana M, Liu X, Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem. 2003; 278:22424–22431.

12. Tsushima H, Okazaki K, Hayashida M, Ushijima T, Iwamoto Y. CCAAT/enhancer binding protein β regulates expression of matrix metalloproteinase-3 in arthritis. Ann Rheum Dis. 2012; 71:99–107.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download