Abstract
Background
Parallel-polarized light (PPL) photography evaluates skin characteristics by analyzing light reflections from the skin surface.
Objective
The aim of this study was to determine the significance of quantitative analysis of PPL images in rosacea patients, and to provide a new objective evaluation method for use in clinical research and practice.
Methods
A total of 49 rosacea patients were enrolled. PPL images using green and white light emitting diodes (LEDs) were taken of the lesion and an adjacent normal area. The values from the PPL images were converted to CIELAB coordinates: L* corresponding to the brightness, a* to the red and green intensities, and b* to the yellow and blue intensities.
Results
A standard grading system showed negative correlations with L* (r=−0.67862, p=0.0108) and b* (r=−0.67862, p=0.0108), and a positive correlation with a* (r=0.64194, p=0.0180) with the green LEDs for papulopustular rosacea (PPR) types. The xerosis severity scale showed a positive correlation with L* (r=0.36709, p=0.0276) and a negative correlation with b* (r=−0.33068, p=0.0489) with the white LEDs for erythematotelangiectatic rosacea (ETR) types. In the ETR types, there was brighter lesional and normal skin with white LEDs and a higher score on the xerosis severity scale than the PPR types.
Parallel-polarized light (PPL) photography is a method that can objectively evaluate reflections from the skin surface1. A preliminary study with various skin diseases indicated that PPL photography images taken with green light emitting diodes (LEDs) might be useful for analyzing specific diseases such as atopic dermatitis, rosacea, and xerotic dermatitis2. Based on our previous study, this study was designed to statistically correlate the CIELAB coordinates with rosacea severity. The L* coordinate represents brightness, and the a* and b* coordinates represent the red to green axis and the blue to yellow axis, respectively3. In the future, clinical research on rosacea could apply this method as an objective assessment of disease severity.
This study was approved by the Institutional Review Board (IRB) of Korea University Anam Hospital (IRB ED13197). A total of 49 patients who visited the clinic between June and July 2015 were enrolled. Twenty-five were men and 24 were women. The mean age was 50.7±14.3 years (range, 17~78 years). Patients with severe medical conditions such as malnutrition, thyroid disorders, or malignancy were excluded. Patients who were using a medication that might affect skin measurements such as diuretics, steroids, retinoids, or H2 antihistamines were excluded. We also excluded pregnant women.
A high intensity power LED was used as a light source. A circuit was assembled with a radiator and constant current LED driver using white (PP00W-8L61-ESBI; Photron Co. Ltd., Anseong, Korea) and green (PP525-8L61-ESBI; Photron Co. Ltd.) LEDs. The circuit board assembly was operated by a 5 V power supply. A rechargeable battery with a power switch was installed to allow easy access and free movement. A rotatable polarizing filter (PL filter; Kenko Co. Ltd., Tokyo, Japan) was attached at the exit slit of the LED lamp, which enabled control of the polarization direction. A digital single-lens reflex camera (EOS-500D; Canon Inc., Tokyo, Japan) equipped with a macro lens (SP MF 90 mm F/2.8 Di Macro 1:1, Tamron Co. Ltd., Saitama, Japan) was used, and a rotatable polarizing filter was placed over the camera lens. The distance between the camera and the subject was maintained constant by using a manual body focusing technique.
The polarizing filters of both the LED lamp and camera were aligned in the same direction. The white and green LED illuminators were attached on each side of the camera at a 45 degree angle as shown in Fig. 1. The white balance of the camera was set to daylight on manual mode with F-number 2.5, shutter speed 1/60 s, and ISO 1600. The subject was placed about 9 cm from the camera to maintain focused images. Only the green or white LED illuminator was turned on in a darkroom to minimize environmental impacts on the images. The PPL images of the skin lesion and adjacent normal appearing skin were taken at the same time.
The PPL digital images were represented using 8-bit integer numbers of sRGB coordinates using a graphic program (Adobe Photoshop Element; Adobe System Inc., San Jose, CA, USA). These code values were converted to CIELAB coordinates using a spreadsheet tool (Excel 2010; Microsoft Corp., Redmond, WA, USA). The CIELAB coordinates of L*, a*, and b* allowed quantitative characterizations of changes in color.
The clinical severity was assessed with a 5 point investigator's global assessment (IGA) scale. All patients were also evaluated with the xerosis severity scale (from 0 to 6)4. We also used the standard grading system proposed by the National Rosacea Society Expert Committee to evaluate rosacea severity5. Relationships between the disease severity index, xerotic severity scale, age, and CIELAB coordinates were examined (Table 1). We also analyzed the data according to sex and rosacea type (Table 2, 3). Patients were classified according to clinical appearance into the erythematotelangiectatic rosacea (ETR) and papulopustular rosacea (PPR) subtypes.
The data were analyzed using the statistical software IBM SPSS Statistics ver. 20.0 for Windows (IBM Co., Armonk, NY, USA). All values were evaluated using both Pearson correlation and Spearman correlation analyses as parametric and non-parametric tests, respectively. The ordinal scales such as the xerosis severity scale were compared between groups using the Mann-Whitney U-test. A p-value <0.05 was considered statistically significant.
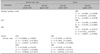
Table 1 shows the relationships between the disease severity index, xerotic severity scale, age, and CIELAB coordinates. The severity of rosacea using the IGA score had a significant positive correlation with the standard grading system proposed by the National Rosacea Society Expert Committee (r=0.79791, p<0.001). For the rosacea skin lesions, the standard grading system showed negative correlations with the L* (r=−0.33348, p=0.0192) and b* coordinates (r=−0.36298, p=0.0104), and a positive correlation with the a* coordinate (r=0.34004, p=0.0168) using green PPL images. In the same environment, there was no correlation for the normal skin area for L* (r=0.10488, p=0.4733), a* (r=−0.11061, p=0.4493), or b* (r=0.06473, p=0.6586).
For white PPL images, the xerosis severity scale showed a positive correlation with L* (r=0.40175, p=0.0042) and a negative correlation with b* (r=−0.29714, p=0.0381) for rosacea lesions. The same correlation was found for normal skin using white PPL images with L* (r=0.52382, p=0.0001), b* (r=−0.33021, p=0.0205).
Age showed a negative correlation with the L* (r=−0.49428, p=0.0003) and b* coordinates (r=−0.46273, p=0.0008), and a positive correlation with the a* coordinate (r=0.49321, p=0.0003) for the rosacea lesion using green PPL images. A similar correlation was found for age and normal skin area using green PPL images, with L* (r=−0.33514, p=0.0186), a* (r=0.34678, p=0.0146), and b* (r=−0.30251, p=0.0346).
The analysis based on sex is shown in Table 2. When analyzed separately by sex, the severity of rosacea using the IGA score had significant positive correlations with the standard grading system in both male (r=0.83617, p<0.0001) and female patients (r=0.75593, p<0.001). The standard grading system for rosacea among the male patients had negative correlations with the L* (r=−0.50907, p=0.0094) and b* coordinates (r=−0.54441, p=0.0049), and a positive correlation with the a* coordinate (r=0.52085, p=0.0076) using green PPL images. However, the standard grading system for the female patients did not show any significant correlation with the L* (r=−0.12598, p=0.5575), a* (r=0.14081, p=0.5117), or b* coordinate (r=−0.14081, p=0.5117).
The age of the male patients was negatively correlated with the L* (r=−0.54783, p=0.0046) and b* coordinates (r=−0.50202, p=0.0106) and positively correlated with the a* coordinate (r=0.53898, p=0.0054) using green PPL images. In the same environment, no significant relationship was found between the age of the female patients and the CIELAB coordinates, with L* (r=−0.33660, p=0.1078), a* (r=0.33355, p=0.1112), and b* (r=−0.32920, p=0.1162).
The analysis based on the clinical subtypes is shown in Table 3. The IGA score of the PPR type had negative correlations with the L* (r=−0.55594, p=0.0485) and the b* coordinate (r=−0.55594, p=0.0485) for the rosacea lesions using green PPL images. The standard grading system showed negative correlations with the L* (r=−0.67862, p=0.0108) and the b* coordinate (r=−0.67862, p=0.0108) and a positive correlation with the a* coordinate (r=0.64194, p=0.0180) for the green LED only with the PPR type. Age also had negative correlations with the L* (r=−0.66391, p=0.0133) and b* coordinates (r=−0.68871, p=0.0092) and a positive correlation with the a* coordinate (r=0.72452, p=0.0051) for the green LED with the PPR type.
The xerotic severity scale showed a positive correlation with L* (r=0.36709, p=0.0276) and a negative correlation with the b* coordinate (r=−0.33068, p=0.0489) in the white LED only for the ETR type. The xerotic severity scale had the same correlations with L* (r=0.49009, p=0.0024) and b* (r=−0.43852, p=0.0075) for the normal skin area using white LEDs with the ETR type. White LEDs showed a higher L* value for ETR than for PPR, which means more brightness, for both the lesional (p=0.0358) and normal skin (p=0.0092). It was notable that the xerosis severity scale score was higher, indicating drier, in the ETR type than the PPR type (p=0.0287).
Many previous publications evaluated skin lesions based on direct visualization and standard flash photography. This kind of assessment often relies on some degree of subjective interpretation and different parameters affect any objective measure. Direct visualization and clinical photography can have different evaluations depending on the observer. Conventional photography may also be taken differently due to inconsistent framing, varying angles between the subject and the camera, and changes in exposure settings.
In order to improve diagnostic evaluation, various non-invasive devices such as ultraviolet light photography, polarized light photography, reflectance spectroscopy, dermoscopy, and confocal scanning laser microscopy have been developed and are widely used clinically6. Among these, polarized light photography is a well-known technique that uses the polarization of the light reflected from skin tissues. It can be applied to evaluate skin diseases such as acne vulgaris, photoaging, subclinical actinic keratosis, nonmelanoma skin cancer, subclinical levels of skin irritant reactions, and psoriasis78910.
Light reflected from the skin can be divided into regular reflectance related to the skin surface and “back-scattered” light from structures within the skin tissue. The regular reflectance is a reflection at the stratum corneum-air interface. The “back-scattered” light originates from the papillary dermis. Light particles reflected from the stratum corneum-air interface have the same polarization direction of the incident light, whereas particles reflected from the papillary dermis have the scattering direction of polarization. Therefore, an observer can use the polarizing light source to selectively examine the surface or subsurface components of the skin1112. When a polarizing filter attached to the camera is aligned parallel to the direction of polarization of the LED, information about the skin surface except the “back-scattered” light component can be obtained. Information on the subsurface component can be obtained by using perpendicular polarized photography.
Therefore, parallel polarized photography can reduce intracutaneous and subsurface details such as pigmentation, vascularity, and color, while emphasizing skin surface detail such as texture and elevation scaling6. Despite these advantages, PPL images are not widely used in clinical practice due to challenges in quantitative assessment. Previous studies indicated that the CIELAB values from the PPL photographic images can serve as a quantitative indicator of skin surface reflection13. In our previous study, the degree of severity and dryness of skin had significant correlations with CIELAB values from the images taken from PPL photography using green LEDs, particularly those with atopic dermatitis, rosacea, and xerotic dermatitis2.
Unlike other noninvasive equipment like ultraviolet photography or dermoscopy, the CIELAB coordinates obtained from PPL photography are relatively consistent parameters regardless of the technique used or the physician's experience. This study was conducted on patients with rosacea. Erythematous lesions appear black, while normal skin appears white using light emitting from a green LED10. The scale of whiteness is especially clearly visualized by PPL photography10.
Skin color is a result of complex interactions of skin microstructures and chromophores with the incipient light. One of the most important chromophores of human skin is hemoglobin, which is located in the lumen of vessels. If the skin color measuring device is pushed against the skin, then the amount of hemoglobin changes and skin color as well. Therefore, most contact type colorimetric devices have drawbacks that cannot be overcome easily. In this study, we used a technique modified from colorimetric photography, a non-contact type measurement that does not affect the vessels and, hence, does not affect erythema.
We observed significant relationships between the standard grading system for rosacea and the CIELAB coordinates, especially using the green PPL images (Table 1). However, male and female patients also displayed important differences. Only the male rosacea patients had significant results on the green LED (Table 2). Also, in the ETR and PPR subject groups, the coordinates of the L*, a*, and b* had significant relationships with the standard grading system on the green LED only for the PPR type. No significant correlation with the ETR type was observed. The ETR type showed significant associations with both the xerosis severity scale and the L* and b* coordinates using the white LED (Table 3).
Only male subjects had a significant association with the severity score of the disease and the L*, a*, and b* coordinates, which may be due to a more severe form of the disease in male patients. This may be related to clinical evidence that rosacea occurs more frequently in middle aged women, but severe symptoms appear more frequently in men. In addition, most patients enrolled in this study had mild to moderate disease. If additional patients or patients with more severe complications are enrolled, the standard grading system in female patients may become significantly correlated with the CIELAB coordinates. The significant relationship between the standard grading system for only the PPR patients and the CIELAB coordinates may also be due to more severe forms of the PPR type enrolled in the study. The standard grading system of the ETR subjects could have a significant correlation if more severe cases of the ETR form were recruited.
The clinical differences between the PPR and ETR subtypes appeared to be relevant in the pathophysiology. As shown on Table 3, dryness of the skin could be an important factor in the pathophysiology of the ETR type while age is more involved with the PPR type. The results in Table 3 indicate that ETR subtypes are observed more precisely using white LEDs and that of PPR subtypes were better evaluated using green LEDs. ETR subtypes were more associated with dryness and brighter (a higher L* value) reflections than PPR subtypes. The results of this study could be used in clinical differentiation of the two subtypes, resulting in more active and rapid treatment. Further studies should examine the mixed type of the PPR and ETR subtypes.
Sun exposure, temperature changes, cytokines and chemokines, various microbial agents, neuroimmune and immune defense connections, angiogenesis, lymphangiogenesis, and other known factors contribute to the pathophysiology of rosacea14. Other factors involved in the pathophysiology of rosacea should be studied further. The results of this study will help us to detect pathophysiological differences between certain subtypes of rosacea.
PPL photography is a method that can objectively evaluate reflections from the skin surface. The CIELAB coordinates from green PPL photography showed significant correlations with the severity index of rosacea. It can be used in clinical settings and may contribute to revealing the pathophysiology of rosacea. In addition, it seems that subtypes of rosacea, e.g., ETR and PPR, are distinct entities visually and optically.
Figures and Tables
Fig. 1
A schematic diagram showing the arrangement of the equipment. LED: light emitting diode, PL: polarized light.
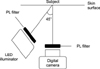
Table 1
Overall results

Table 2
Analysis by men and women

Table 3
Analysis by ETR and PPR subtypes
ACKNOWLEDGMENT
Supported by the Korea Research Foundation Grant funded by the Korean Government (NRF-2012R1A1A2044700).
References
1. Kim DH, Choi JE, Ryu HJ, Seo SH, Kye YC, Ahn HH. Analytic parallel-polarized light imaging technique using various light-emitting diodes: a comparison with skin conductance values. Skin Res Technol. 2015; 21:158–163.

2. Kye H, Choi JE, Seo SH, Kye YC, Ahn HH. Application of analytic technique using green light parallel-polarized light images in various skin diseases. Ann Dermatol. 2016; 28:242–245.

3. Latreille J, Gardinier S, Ambroisine L, Mauger E, Tenenhaus M, Guéhenneux S, et al. Influence of skin colour on the detection of cutaneous erythema and tanning phenomena using reflectance spectrophotometry. Skin Res Technol. 2007; 13:236–241.

4. Rogers RS 3rd, Callen J, Wehr R, Krochmal L. Comparative efficacy of 12% ammonium lactate lotion and 5% lactic acid lotion in the treatment of moderate to severe xerosis. J Am Acad Dermatol. 1989; 21:714–716.

5. Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, et al. Standard grading system for rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004; 50:907–912.

6. Taylor S, Westerhof W, Im S, Lim J. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol. 2006; 54:5 Suppl 2. S282–S290.

7. Phillips SB, Kollias N, Gillies R, Muccini JA, Drake LA. Polarized light photography enhances visualization of inflammatory lesions of acne vulgaris. J Am Acad Dermatol. 1997; 37:948–952.

8. Farage MA. Enhancement of visual scoring of skin irritant reactions using cross-polarized light and parallel-polarized light. Contact Dermatitis. 2008; 58:147–155.

9. Ortonne JP, Gupta G, Ortonne N, Duteil L, Queille C, Mallefet P. Effectiveness of cross polarized light and fluorescence diagnosis for detection of sub-clinical and clinical actinic keratosis during imiquimod treatment. Exp Dermatol. 2010; 19:641–647.

10. Kollias N, Stamatas GN. Optical non-invasive approaches to diagnosis of skin diseases. J Investig Dermatol Symp Proc. 2002; 7:64–75.

11. Anderson RR. Polarized light examination and photography of the skin. Arch Dermatol. 1991; 127:1000–1005.

12. Muccini JA, Kollias N, Phillips SB, Anderson RR, Sober AJ, Stiller MJ, et al. Polarized light photography in the evaluation of photoaging. J Am Acad Dermatol. 1995; 33:765–769.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download