Abstract
Background
Alopecia areata (AA) is a T cell-mediated autoimmune disease that targets hair follicles and interrupts hair regrowth. The microenvironment of the effector T cells and their related cytokines may affect immunopathogenesis around the hair bulb/bulge.
Objective
To determine the contributory roles of the effector T cell subsets and related cytokines to the pathogenesis of AA.
Methods
We investigated the correlation between histopathological grades and four clinical prognostic factors in 331 patients with AA, and analyzed the topography of T cell infiltrates and related cytokines around the hair bulb/bulge according to histopathological grades through immunohistochemical and double immunofluorescence studies on a subset of AA specimens.
Results
First, the groups with more severe histopathological grades were associated with earlier onset, longer duration, more hair loss, as well as poorer therapeutic outcomes. Second, the pattern of CD4 and CD8 expression around the hair bulb/bulge varied by histopathological grade, with staining density decreasing in the following order: type 1>type 2>type 3. In addition, interferon-γ and transforming growth factor-β1 expression appeared denser in the peribulbar area. Interestingly, the denser CCR6+ cells (Th17 cells) showed more infiltration than CCR5+ cells (Th1 cells) around the hair bulb/bulge as histopathological grade worsened.
Conclusion
The insidious destruction of bulge stem cells and hair bulb matrix stem cells results in more severe hair loss in patients with chronic AA, which is mediated by Th17 lymphocyte and cytotoxic T lymphocyte infiltration. Furthermore, Th17 lymphocytes may play an even more important role than cytotoxic T cells in the development of AA.
Alopecia areata (AA) is a T cell-mediated autoimmune disease that targets hair follicles. Collapsed immune privilege in hair follicles may play a crucial part in the pathogenesis of AA1. Interferon (IFN)-γ secretion is triggered by infection, bacterial superantigens, psycho-emotional stressors, skin microtrauma, or other damage to hair follicles and collapse of the immune privileged state, which is maintained by α-melanocyte-stimulating hormone (α-MSH), transforming growth factor (TGF)-β1 and insulin-like growth factor-I that upregulates major histocompatibility complex (MHC) class I expression in hair follicles. Because MHC class I molecules are strongly expressed in AA lesions, CD8+ T cells react to autoantigens in the hair follicles2. In the event that a given individual has pre-existing autoreactive CD8+ T cells, which must receive appropriate co-stimulatory signals and receive help from CD4+ T cells, a cytotoxic T cell attack is launched on the hair matrix. This attack activates a vicious circle of secondary, follicle-damaging autoimmune phenomena, whose quality and magnitude largely determine the degree of resulting hair follicle damage (dystrophy) and thus the clinical manifestations and progression of AA. Some authors have recently proposed that AA may be associated with Th17 cells, in addition to Th1 cells. However, their contributory roles in AA pathogenesis have not been elucidated to date3. Tanemura et al.4 demonstrated infiltration of CD4+interleukin (IL)-17A+Th17 cells in the dermis, particularly around the hair follicles in patients with AA. Lew et al.5 showed that an IL17RA gene polymorphism might contribute to increased susceptibility to AA in the Korean population, and that the IL17RA gene polymorphism may be associated with age of AA onset.
Although the bulge region is not affected by an autoimmune response in AA because of ‘non-scarring’ alopecia4, T lymphocytes sometimes infiltrate into the bulge region as well as the intra- and peri-bulbar areas67. Inflammatory injury involves the bulbar region of hair follicles and spares the bulge region in early AA lesions, but it does not lead to permanent hair loss8. However, a few patients with AA may lose their ability to grow new hair, and the involvement of stem cells has been suggested in this type of ‘non-scarring’ alopecia9.
Recently, several biomarkers of human bulge cells, including keratin15 (K15), follistatin, and CD200, have been identified. In addition, stem cell involvement was demonstrated in two elderly women with AA and unusual lymphocytic cell infiltrates surrounding both the bulge and the bulbar regions. Both K15 and CD200 immunoreactivities were decreased in the affected bulge lesions of bulge-involving AA, compared to that of the bulge lesions of common AA cases10.
In this study, we identified the contributory roles of various effector T cell subsets, including Th17 cells, Th1 cells, and CD8+ cytotoxic T cells, along with their related cytokines, in the immunopathogenesis of AA.
We retrospectively reviewed 331 cases of patients with AA who had biopsies performed in Dong-A University Hospital between January 1994 and May 2013. We classified these cases into acute, subacute, and chronic groups, according to onset and duration of disease. Chronic AA is defined by a duration of disease that is longer than 3 months at the time of biopsy8. Of the cases, 311 were considered chronic AA. This study was approved by the institutional review board of Dong-A University Medical Center (IRB no. 15-102) and conducted in accordance with the guidelines of the 1975 Declaration of Helsinki. All data were analysed using SPSS ver. 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
We investigated various clinical prognostic factors such as sex, age, disease duration, clinical type, extent of hair loss, and therapeutic outcomes in the patients with AA in this study. In addition, we investigated associations between each clinical prognostic factor and histopathological grades according to the Uno and Orecchia classification. Clinical types of AA were classified by the extent of hair loss: patchy type of AA with single or multiple patches (hair loss <50%), AA subtotal is type (hair loss >50%), AA total is type (affecting all of the scalp), and AA universal is type (hair loss over the whole body). We treated the patients with topical immunotherapy using diphenylcyclopropenone, topical application of cyclosporine (20 mM), triamcinolone acetonide intralesional injections, topical application of corticosteroid, and oral administration of cyclosporine. Therapeutic outcomes were categorized into 6 grades, A5 (100% regrowth), A4 (75%~99% regrowth), A3 (50%~75% regrowth), A2 (25%~50% regrowth), A1 (<25% regrowth), and A0 (no change or progression), by the extent of hair regrowth and degree of reduction in hairless patches.
Histopathological features of hairless patches in patients with AA were categorized into four grades according to the Uno and Orecchia classification scheme. Type 1 was defined as “microfollicles with severe associated changes such as hair disruption, follicular degeneration, and perifollicular inflammation”; type 2 as “microfollicles or medium follicles with severe associated changes”; type 3 as “medium follicles with moderate associated changes such as proliferation of perifollicular fibrous tissue”; and type 4 as “large follicles without any associated changes.”
We conducted immunohistochemistry for CD4, CD8, IFN-γ, TGF-β1, and TGF-β2, and double immunofluorescence for CCR6/CCL20 and CCR6/CCR5 in three patients for each group, types 1, 2, 3, and 4 by the Uno and Orecchia classification. We observed and photographed these sections through light microscopy and immunofluorescence microscopy.
Paraffin blocks for the biopsied specimens from the hairless patches of 9 patients with AA (type 1, n=3; type 2, n=3; type 3, n=3; and type 4, n=0) were cut into 4-µm thick sections and incubated at 58℃ overnight. The paraffin sections were deparaffinized with 100% xylene two times for 10 minutes each and washed with graded concentrations (100%, 90%, 70% and 50%) of ethyl alcohol, followed by application of 0.3% phosphate buffered saline with tween 20 (PBST) solution for 10 minutes and a wash in PBS solution. We rewarmed the sections using 750-watt microwave in 10 mM citrate buffer (pH 6.0) for 5 minutes, followed by warming slides two times for 5 minutes each to retrieve antigens. We cooled sections at room temperature for 20 minutes, and subsequently washed sections using washing buffer solution and blocked endogenous peroxidase with 0.3% H2O2 for10 minutes. The sections were then washed with washing buffer three times and reacted at 45℃ for 10 minutes with non-immune goat serum to remove binding of non-specific proteins. The following primary antibodies were used: monoclonal mouse anti-human CD4 (NCL-CD4-1F6; Novocastra Laboratories Ltd., Newcastle Upon Tyne, UK), monoclonal mouse antihuman CD8 (NCL-CD8-4B11; Novocastra Laboratories Ltd.), rabbit anti-IFN-γ antibody (sc-8308; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), mouse monoclonal anti-TGF-β1 antibody (sc-52893; Santa Cruz Biotechnology Inc.). The sections were reacted with the antibodies at room temperature for 30 minutes. After washing with buffer solution three times, the sections were reacted with secondary antibody (horse radish peroxidase, DAKO Envision Detection kit mouse/rabbit) at room temperature for 30 minutes. After washing with buffer solution, the sections were incubated with 0.025% diaminobenzidine (DAB) solution for a chromogen reaction. Counterstaining was performed with Mayer's hematoxylin, and the slides were observed by optical microscope and photographed.
Paraffin blocks of the biopsied specimens from the hairless patches of 9 patients with AA (type 1, n=3; type 2, n=3; type 3, n=3; and type 4, n=0) were cut into 4-µm thick sections and incubated at 58℃ overnight. The paraffin sections were deparaffinized with 100% xylene two times for 10 minutes each and washed with graded concentrations (100%, 90%, 70%, and 50%) of ethyl alcohol, followed by application of 0.3% PBST solution for 10 minutes, a wash with PBS solution, and then application of 10% blocking solution for 60 minutes. The following primary antibodies were used: mouse monoclonal anti-CCR5 antibody (clone 45549; R&D Systems, Abingdon, UK) for the Th1 fraction, and rabbit polyclonal anti-CCR6 antibody (clone ab78429; Abcam, Cambridge, UK) for the Th17 fraction. These primary antibodies were applied to slide sections at 4℃ and incubated overnight. After slide sections were washed with PBS solution, Cy3-conjugated anti-mouse IgG (Invitrogen, CA, USA), Alexa Fluor 594-conjugated anti-goat IgG (Invitrogen) or Oregon Green 488-conjugated anti-rabbit IgG (Invitrogen) were applied as secondary antibodies at 22℃ for 3 hours. Subsequently, after washing with PBS solution, the slides were observed using confocal microscope (LSM 510; Carl Zeiss, Jena, Germany) and photographed.
Among 331 patients, 162 patients were men and 169 patients were women. The mean age was 32.3 years. According to the Uno and Orecchia classification system, 106 (32.0%) patients were categorized as type 1, and 149 (45.0%) and 76 (23.0%) patients were classified as type 2 and 3, respectively. The ages of onset ranged from 1 year to 76 years, and the ages of onset according to histopathological grade were 29.9, 34.4, and 35.8 years in types 1, 2, and 3, respectively. Hence, the histopathological grades showed an inverse correlation with age of onset (p=0.027; Table 1). The duration of disease was 25.1 months on average, and the durations of disease according to histopathological grade were 29.5, 23.1, and 21.4 in types 1, 2, and 3, respectively. Hence, the histopathological grades showed a positive correlation with age of onset (p=0.002; Table 1). We categorized the extent of hair loss as S1~S5 (extent of hair loss, S1: <5%, S2: 5%~25%, S3: 25%~50%, S4: 50%~75%, S5: ≥75%). The type 1 group showed the highest grade of hair loss (23.6% of patients were classified as S5), and the Type 3 group showed the lowest grade (13.2% of patients were classified as S5). The extent of hair loss showed a positive correlation with histopathological grade (p<0.001; Table 1). Therapeutic outcomes were 57.1%, 69.1% and 78.2% in types 1, 2, and 3, respectively. Histopathological grades showed a negative correlation with therapeutic outcomes (p=0.020; Table 1). Most patients (311/331, 94.0%) were classified as chronic AA cases. Patients with chronic AA showed more extensive hair loss and less response to treatment than patients with acute and subacute AA (data not shown). In the histopathological examination, patients with chronic AA also showed greater severity of disease (data not shown).
Immunohistochemistry for CD4 and CD8 revealed peri- or intra-bulbar infiltrations of lymphocytes; CD4+ cells showed peribulbar infiltration in the dermis, whereas CD8+ cells infiltrated at the intra bulbar area. The group with more severe histopathology showed a denser pattern of expression of CD4 and CD8 around the hair bulb (Bb) in decreasing order (type 1>type 2>type 3; Fig. 1). Moreover, we observed, especially at the hair bulge (Bg) region, that CD4+ and CD8+ T lymphocytes showed greater infiltration with histopathological grades associated with more severe disease (Fig. 2).
By double immunofluorescence, CCR6+ lymphocytes infiltrated the intra- or peri-bulbar areas (Bb) in a denser pattern with greater histopathology (Fig. 3), and CCL20 was expressed in epithelial cells of the hair bulb. CCR5+ lymphocytes also infiltrated at intra- or peri-bulbar areas (Bb) in a denser pattern with greater histopathology (Fig. 4). More specifically, CCR6+ lymphocytes also infiltrated at the bulge region (Bg), and with greater histopathology showed denser infiltration of CCR6+ lymphocytes (Fig. 5).
IFN-γ+ cells infiltrated the dermis around the hair bulb and the more severe disease groups by histopathological grade showed denser expression of IFN-γ (Fig. 6). TGF-β1 was expressed in the epithelia of the outer root sheath, regressive epithelial strand, fibrous dermal sheath, and dermal sheath cells induced by inflammatory changes. The more severe disease group by histopathological grade also showed denser expression of TGFβ-1 (Fig. 7). Interestingly, TGF-β2 was expressed primarily in the dermal papilla area, and the more severe disease group by histopathological grade showed less expression of TGF-β2 as the size of the dermal papilla area decreased (Fig. 8).
In the present study, as reported elsewhere regarding clinical prognostic factors in AA, earlier onset of age (p=0.019), longer duration of disease (p=0.018), greater extent of hair loss (p<0.001), and poorer therapeutic outcomes (p<0.001) were significantly correlated with greater histopathology (lower histopathological grades) in our study. Histopathological grades have been recommended as an independent clinical prognostic factor in AA. Most patients (94.0%, 311/331 patients) were classified as having chronic AA, based on the duration of onset. Patients with chronic AA showed more extensive hair loss and a poorer response to treatment than patients with acute and subacute AA. Chronic AA patient also showed greater histopathology, as define by the Uno and Orecchia classification scheme.
Among the various components contributing to histopathological grades, inflammatory infiltrates can lead to other changes such as destruction of hair follicular structures and perifollicular fibroplasia. Consequently, CD8+ T cells as well as CD4+ T cells act as central components in the pathogenesis of AA, and our study indicated that CD4+ T cells mainly infiltrated the peribulbar area in the dermis and CD8+ T cells mainly infiltrated the intrabulbar area, similar to what has been reported in previous studies. More specifically, denser peribulbar and intrabulbar infiltration of T cells with greater histopathology were observed in this study. Furthermore, in this study, the denser CCR6+ putative Th17 cells infiltrated the hair bulbs in AA hair follicles.
Although it is widely known that the bulge area is not affected by an autoimmune response in AA4, some authors recently reported that T cells could infiltrate the bulge region67. We demonstrated also in this study that the denser T cell (CD4+ T and CD8+ T cells) infiltrates at the bulge region were associated with greater histopathology11, and that denser CCR6+ putative Th17 cells infiltrated the hair bulge region primarily in the histopathological grades associated with more severe disease in AA hair follicles (Fig. 5). If the infiltrating T cells such as CD4+ (including CCR5+ Th1 and CCR6+ Th17 cells) and CD8+ T cells attack the bulge stem cells, the hair follicles lose their ability to grow new hairs, and growth of hair in the follicle is interrupted during chronic AA pathogenesis, resulting in greater histopathology.
Recently, a study of the pathogenic role of Th17 cells focused on autoimmune inflammatory diseases such as multiple sclerosis, rheumatoid arthritis, and psoriasis12. AA is characterized by a Th1 response, although the involvement of Th17 has not been elucidated13. CD8+ T cells are considered the direct modulators of hair loss, whereas CD4+ CD25− T cells play a classic helper role in AA onset. Th17 cells are involved in the promotion of autoimmunity, and Treg cells are involved in the control of Th17 cells. Consequently, the balance of Th17/Treg cells is believed to be important in the control of immunity mediated by Th17 cells11. Recently, Th17 cells and Th1 cells were proposed to be associated with AA pathogenesis4. In this study, we found that Th17 cells as well as Th1 cells infiltrated in an admixed pattern in the bulb and bulge areas of AA hair follicles. Th17 and Th1 cells may both play helper roles in AA.
The denser infiltration of Th17 cells than Th1 cells in this study may be explained by the long survival of Th17 cells in chronic inflammation. Acosta-Rodriguez et al.11 showed that Th17 cells, unlike Th1 cells, mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. Th17 cells may be involved in chronic conditions in humans that are analogous to long-lasting inflammation induced by Th17 cells. Here, we found that the denser Th17 cells infiltrated with great histopathology in parallel with a longer duration of disease. These findings may reflect the chronic inflammation in severe AA.
Cytokines are also involved in AA pathogenesis. These cytokines have an important role in the collapse of immune privilege and initiation of signal transduction. Among these cytokines, IFN-γ is one of main cytokines to induce AA via Th1 immunity; it is released by T cells and antigen presenting cells that infiltrate the hair bulbs and bulges14. After autoantigens such as MHC molecules are expressed under the influence of IFN-γ, immune privilege collapses and hair growth is interrupted151617. In previous studies, high serum IFN-γ levels correlated with more severe clinical features. Therefore, IFN-γ can serve as a prognostic factor that reflects the inflammatory state of AA1819. In contrast, the correlation between in situ IFN-γ expression in AA lesions with the histopathological grades has not been determined. In this study, we showed that IFN-γ+ cells were expressed with histopathological grades associated with more severe disease. In the process of AA generation, targeted cell killing by CD8+ cytotoxic T cells with the help of CD4+ T helper cells reflects mainly a Th1-mediated immune response, which is usually dependent on the production of IFN-γ20.
Potent immunosuppressants such as TGF-β1 and α-MSH are prominently expressed in the proximal anagen hair follicle21. TGF-β1 is considered an immunosuppressant that helps maintain immune privilege by inhibiting expression of autoantigens in the follicular epithelia. TGF-β1 is expressed in the hair bulb in the early anagen period and in the outer root sheath and degenerative epithelial strand in the transition period (anagen to telogen), and inhibits follicular epithelial proliferation, resulting in apoptosis and telogen induction2223. In this study, TGF-β1 was expressed in the outer root sheath and degenerative epithelial strand in the transition period and also in the fibrous dermal sheath and dermal sheath cells with inflammation. These results suggest that TGF-β1 has an important role in AA pathogenesis in inducing catagen hair and perifollicular fibrosis by modifying the extracellular matrix.
In the normal hair cycle, TGF-β2 is also expressed in the follicular outer root sheath in the anagen period. TGF-β2 is expressed in the hair matrix and degenerating follicular strands in the transition period. In the presence of neutralizing antibody, as well as a TGF-β antagonist, hair follicles were markedly elongated in a concentration-dependent manner. Elongation of the hair follicle was significantly suppressed by TGF-β2. These results strongly suggest that TGF-β2 plays an essential part in the induction of the catagen phase of the human hair cycle24. However, in contrast to results from previous studies, TGF-β2 was not found to be expressed on the degenerative follicular epithelia in this study.
Interestingly, TGF-β2 may also play an important role in the genesis of hair follicles because it is expressed on the hair papilla25. The dermal papilla is the most important mesenchymal component in hair follicle formation, and it has an essential role in follicular proliferation through its interaction with the epithelia of hair matrix (mesenchymal epithelial interaction). In our study, TGF-β2 was expressed in the dermal papilla area, and its expression was inversely correlated with the histopathological grade and with size of the dermal papilla area.
In conclusion, we identified the role of effector T cell subsets and cytokines, including IFN-γ and TGF-β, in AA pathogenesis. As in previous studies, CD4+ T cells mainly infiltrated the peribulbar area in the dermis, whereas CD8+ T cells mainly infiltrated the intrabulbar area, and the denser T lymphocytes infiltrated with greater histopathology at hair bulbar area (Bb). Further, we also observed that T cells (CD4+ and CD8+ T cells) infiltrated the bulge region in the groups with histopathological grades associated with more severe disease. Therefore, we hypothesizes that severe hair loss in patients with chronic AA may result from the destruction of bulge stem cells in the bulge region as well as hair matrix stem cells in the bulb area. In addition, Th17 immunity plays an important role other than Th1 immunity in the development of AA. IFN-γ+ cells infiltrated into the dermis around the hair bulb, and the histopathological grades associated with more severe disease showed more expression of IFN-γ as a putative prognostic factor. TGF-β2 was expressed primarily in the dermal papilla area and in histopathological grades associated with more severe disease, with the expression of TGF-β2 inversely correlated with the size of dermal papilla area, reflecting interrupted folliculogenesis in an inflammatory condition such as AA.
Figures and Tables
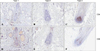 | Fig. 1Immunnohistochemistries for CD4+ and CD8+ lymphocytes. CD4 positive cells infiltrated mostly in peribulbar area. CD8+ cells infiltrated mostly in intrabulbar area. The severer histopathologic gradings were associated with the denser infiltrations of mononuclear cell (A~C: CD4, ×100), (D~F, CD8, ×100). |
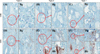 | Fig. 2CD4 and CD8 immunohistochemistries for types 1, 2, and 3 hair follicles in alopecia areata (AA) patients: (A) and (D), type 3 AA hair follicles, (B) and (E); type 2 AA hair follicles, (C) and (F); type 1 AA hair follicles. At hair bulb (Bb) areas, CD4+ T lymphocytes infiltrated perifollicularly and CD8+ T lymphocytes infiltrated intrafollicularly, Interestingly, the denser CD4+ and CD8+ T lymphocytes infiltrated around hair bulge (Bg) regions as the histopathologic gradings were worsened. |
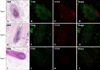 | Fig. 3Expression of CCR6 and CCL20 within type 1 hair bulbs (B~D), type 2 hair bulbs (F~H), and type 3 hair bulbs (J~L). A representative finding of double immunofluoroscence showed immunoreactive cells for CCR6 (B, F, J; green) and CCL20 (C, G, K; red) withco-localization study of CCR6 and CCL20 (D, H, L). Same samples for H&E stain were also shown from type 1 to type 3 (A, E ,I). The denser CCR6 and CCL20 were expressed in parallel with the severer histopathologic gradings (H&E: A, E, I, ×100; double immunofluorescence: B~D, F~H, J~L, ×100). |
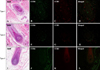 | Fig. 4Expression of CCR6 and CCR5 within type 1 hair bulbs (B~D), type 2 hair bulbs (F~H), and type 3 hair bulbs (J~L). A representative finding of double immunofluorescences immunoreactive cells for CCR6 (B, F, J; green) and CCR5 (C, G, K; red) with colocalization study of CCR6 and CCR5 (D, H, L). H&E stained sectionswere also shown from type 1 to type 3 (A, E, I). The denser CCR6 and CCR5 were expressed in parallel with the severer histopathologic gradings (H&E: A, E, I, ×100; double immunofluorescence, B~D, F~H, J~L, ×100). |
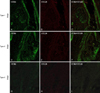 | Fig. 5Expression of CCR6 and CCL20 within type 1 hair bulges (A~C), type 2 hair bulges (D~F), and type 3 hair bulges (G~I). |
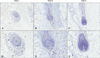 | Fig. 6Immunnohistochemistry for interferon (IFN)-γ. IFN-γ wasexpressed in the inflammatory cells around the hair bulb, and the severer histopathologic grading was associated with the denser immunoreactive cells for IFN-γ (A~C, ×100; D~F, ×200). |
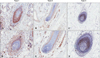 | Fig. 7Immunnohistochemistry for transforming growth factor (TGF)-β1. Expression of TGF-β1 was detected in the outer root sheath keratinocyte, regressing epithelial strand, dermal sheath and dermal sheath cells, and its expression was correlated with histopathologic grading (A~C, ×100; D~F, ×200). |
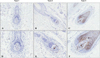 | Fig. 8Immunnohistochemistry for transforming growth factor (TGF)-β2. TGF-β2 was expressed in the dermal papilla area, and less expression was correlated with the severer histopathologic gradings (A~C, ×100; D~F, ×200). |
Table 1
Clinical prognostic factors were correlated with the histopathologic gradings (n=331)

Values are presented as mean±standard deviation or number (%). The severe histopathologic gradings were significantly associated with early age of onset, longer disease duration, greater extent hair loss (extent of hair loss; S1: <5%, S2: 5%~25%, S3: 25%~50%, S4: 50%~75%, S5: ≥75%), poorer response to treatment (extent of hair regrowth; A5: 100%, A4: 75%~99%, A3: 50%~75%, A3: 25%~50%, A1: <25%, A0: no change or progression).
References
1. Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000; 142:862–873.

2. Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008; 159:1077–1085.

3. McElwee KJ, Freyschmidt-Paul P, Hoffmann R, Kissling S, Hummel S, Vitacolonna M, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005; 124:947–957.

4. Tanemura A, Oiso N, Nakano M, Itoi S, Kawada A, Katayama I. Alopecia areata: infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology. 2013; 226:333–336.

5. Lew BL, Cho HR, Haw S, Kim HJ, Chung JH, Sim WY. Association between IL17A/IL17RA gene polymorphisms and susceptibility to alopecia areata in the Korean population. Ann Dermatol. 2012; 24:61–65.

6. Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001; 7:293–301.

7. Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol. 2005; 32:675–679.

8. Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003; 139:1555–1559.
10. Yoshida R, Tanaka K, Amagai M, Ohyama M. Involvement of the bulge region with decreased expression of hair follicle stem cell markers in senile female cases of alopecia areata. J Eur Acad Dermatol Venereol. 2011; 25:1346–1350.

11. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007; 4:639–646.

12. Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-AvelarMde J, García-Magallanes N, Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam. 2014; 2014:651503.

13. Oiso N, Kawada A. Renbök phenomenon in a patient with alopecia areata universalis and psoriasis. J Dermatol. 2012; 39:288–289.

14. König A, Happle R, Hoffmann R. IFN-gamma-induced HLA-DR but not ICAM-1 expression on cultured dermal papilla cells is downregulated by TNF-alpha. Arch Dermatol Res. 1997; 289:466–470.

15. Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Mediation of alopecia areata by cooperation between CD4+ and CD8+ T lymphocytes: transfer to human scalp explants on Prkdc(scid) mice. Arch Dermatol. 2002; 138:916–922.
16. Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. J Invest Dermatol. 2005; 124:288–289.

17. Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003; 8:188–194.

18. Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Derm Venereol. 1996; 76:421–423.
19. Arca E, Muşabak U, Akar A, Erbil AH, Taştan HB. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004; 14:33–36.
21. Paus R, Christoph T, Müller-Röver S. Immunology of the hair follicle: a short journey into terra incognita. J Investig Dermatol Symp Proc. 1999; 4:226–234.

22. Paus R, Foitzik K, Welker P, Bulfone-Paus S, Eichmüller S. Transforming growth factor-beta receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol. 1997; 109:518–526.

23. Welker P, Foitzik K, Bulfone-Paus S, Henz BM, Paus R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor beta 1 and beta 3 in murine skin. Arch Dermatol Res. 1997; 289:554–557.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download