Abstract
The development of cutaneous sarcoidosis as a paradoxical adverse event of tumor necrosis factor alpha (TNF-α) blockers has been reported in the literature; however, an erythrodermic form of cutaneous sarcoidosis during anti-TNF-α therapy has not yet been reported. Herein, we report the first case of an erythrodermic form of cutaneous sarcoidosis during anti-TNF-α therapy and review previous studies of cutaneous sarcoidosis. A 6-year-old Korean girl who had been suffering from juvenile rheumatoid arthritis presented with generalized erythematous skin eruption involving more than about 90% of her body surface area. After 14 months of etanercept treatment, the new erythematous skin eruption had developed and progressed into generalized erythroderma. Exclusion of suspected co-medication had been performed based on medication history. She had no other systemic symptoms, and ophthalmologic and neurologic examinations were normal. Histopathologic findings of the skin lesion revealed diffuse non-caseating granulomatous infiltrates composed of epithelioid histiocytes with sparse lymphocytes involving the entire dermis. Periodic-acid-Schiff and acid-fast stains were negative, and acid-fast bacilli was not detected by polymerase chain reaction of the skin biopsy. Based on clinicopathologic findings, she was diagnosed with etanercept-induced sarcoidal granuloma. After discontinuation of the suspected agent, the lesions spontaneously disappeared.
Tumor necrosis factor alpha (TNF-α) blocker has been used in various rheumatic disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel diseases, and sarcoidosis. The increased use of this agent has been noted in dermatologic diseases including severe recalcitrant psoriasis or psoriatic arthritis1. Extensive use of TNF-α blocker may induce diverse cutaneous adverse effects like infection, systemic or cutaneous lupus erythematosus, or vasculitis. Paradoxically, psoriasis and cutaneous sarcoidosis have been reported as adverse effects of anti-TNF therapy12. New onset of systemic sarcoidosis during treatment with TNF-α blockers is well known, but cutaneous sarcoidosis caused by this agent has been rarely reported in children in the dermatologic literature 345678910111213141516171819. Herein, we report the first case of an erythrodermic form of cutaneous sarcoidosis during anti-TNF-α in juvenile rheumatoid arthritis (JRA) and review TNF-α blocker-induced cutaneous sarcoidosis in previous studies.
A 6-year-old Korean girl presented to us for generalized erythematous skin eruption for about 1 year. Four years prior (December 2011), she had been diagnosed with JRA and was started on methotrexate, sulfasalazine, non-steroidal anti-inflammatory drugs, and prednisolone. One year later (December 2012), in the course of tapering the dose of prednisolone, the symptoms of JRA had been aggravated, and etanercept 25 mg subcutaneous injection twice a week had been newly added to her regimen. In February 2014, 14 months after starting etanercept treatment, a new erythematous skin eruption developed and progressed into generalized erythroderma. In October 2014, all oral medication was withdrawn from her regimen other than etanercept injection, but the skin lesion has persisted without any change. At the first visit to our dermatology department in February 2015, physical examination showed widespread discrete orange to reddish follicular hyperkeratotic papules with scales that had developed into generalized erythroderma involving more than 90% of the body surface area (Fig. 1A, B). She had no other systemic symptoms, and ophthalmologic and neurologic examinations were normal. Laboratory examination including complete blood cell count, routine serum chemistry, total calcium, and angiotensin-converting enzyme level were all within normal limits. Histopathologic findings of the skin lesion revealed diffuse non-caseating granulomatous infiltrates composed of epithelioid histiocytes with sparse lymphocytes involving the entire dermis (Fig. 2). Periodic acid-Schiff and acid-fast stains were negative, and acid-fast bacilli were not detected in the skin biopsy by polymerase chain reaction. Radiologic examination including chest X-ray, computed tomography, and bone scan revealed multiple enlarged lymph nodes in the cervical, both axillary, and both external and internal iliac areas. Based on clinicopathologic findings, she was diagnosed with etanercept-induced sarcoidal granuloma. The etanercept treatment was discontinued and changed to hydroxychloroquine and prednisolone to treat the underlying JRA. In addition to the JRA treatment, we encouraged the patient to use emollients daily. Two weeks after withdrawal of the offending drug, the hyperkeratotic skin lesions started to flatten, and the erythematous color faded. Oral prednisolone was tapered off three months after initiation because there were no signs or symptoms of JRA aggravation. At the five-month follow-up visit after discontinuation of the drug, significant improvements in the skin lesions were observed (Fig. 1C).
The development of cutaneous sarcoidosis as a paradoxical adverse event of TNF-α blockers has been reported in the literature (Table 1)345678910111213141516171819. The exact pathophysiology of the paradoxical event remains unknown; however, it is assumed that the administration of anti-TNF agents to control inflammatory disorders can disturb TNF-α level in the signaling pathway and results in unintended adverse effects9. The most common indication of TNF-α blockers in our case review was rheumatoid arthritis, followed by ankylosing spondylitis. Of them, 14 cases were induced by etanercept, 5 cases by adalimumab, and 5 cases by infliximab. Etanercept has shown a higher frequency of cutaneous sarcoidosis than other TNF-α blockers. Moustou et al.1 explain that the different mechanisms of action between etanercept and infliximab can cause different TNF-α concentrations in tissue, and higher levels of biologically active TNF-α result from etanercept therapy, which may foster the formation of granuloma. Overall, there is female preponderance in the reported cases, and most are adults, except one 7-year-old boy with juvenile idiopathic arthritis4. Our case is the second child case of new onset of cutaneous sarcoidosis induced by TNF-α blocker. The time lapse between TNF-α blocker and cutaneous symptoms appeared to be variable from 1 month to 5 years, and about half of the cases had developed 12 months after initiation of the drug. In our patient, erythrodermic skin eruption emerged 14 months after the first etanercept injection, and subcutaneous injection was continued due to unawareness of its adverse skin eruption. Cutaneous manifestations due to TNF-α blockers are similar to those of classical cutaneous sarcoidosis, presenting most commonly as subcutaneous nodules, erythema nodosum and less commonly as papules, plaque, and scars. Erythrodermic sarcoidosis is a very rare clinical manifestation of cutaneous sarcoidosis appearing as generalized erythematous scaling patches or brown-red sheets; the variant is seldom considered in the clinical differential diagnosis of erythroderma2. All reported cases have shown characteristic histopathologic findings of non-caseating granuloma in the dermis, with a sparse rim of surrounding lymphocytes. In cases of erythrodermic sarcoidosis, as in our case, the dermis shows more diffuse involvement than most typical cases of cutaneous sarcoidosis, with multiple small granulomas and inflammatory infiltrate that is predominantly perivascular and periadenexal20. In most previous cases, clinical recovery was achieved after discontinuation of anti-TNF agents with or without prednisone use. The range of recovery time was variable from 1 to 9 months, but it mainly resolved within 6 months. In our patient, fast recovery was shown 2 weeks after withdrawal of TNF-α blocker. In our case, clinico-histopathological evaluation and exclusion of suspected co-medication was performed, and a strong relationship between TNF-α blocker and skin eruption was found. Clinical remission upon drug cessation was also observed.
In conclusion, we report the first pediatric case of erythrodermic cutaneous sarcoidosis associated with etanercept. As anti-TNF biologic agents are more frequently used in clinical settings, dermatologists should expect to encounter patients with cutaneous side effects after receiving these medications. In addition, atypical and rare forms of clinical manifestations of sarcoidosis including erythroderma should be considered as possible side effects of TNF-α blocker.
Figures and Tables
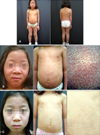 | Fig. 1(A) Monomorphous follicular dull reddish papules fused into generalized erythroderma involving the face, trunk, and both upper and lower extremities. (B) Close-up view of the face and chest with scaly follicular hyperkeratotic papules forming erythrodermic patches. (C) Close-up view of the face and chest at 5 months after cessation of etanercept therapy, showing significant improvement |
 | Fig. 2(A) Lower-magnification view showing diffuse involvement with non-caseating granulomatous infiltrates in the entire dermis (H&E, ×40). (B) Higher-magnification view of dermal infiltrate showing epithelioid cells with sparse lymphocytes (H&E, ×200). |
Table 1
Previously reported and present cases of cutaneous sarcoidosis that developed during anti-tumor necrosis factor-alpha therapy

| Case | Causative agent | Authors | Age(yr)/sex | Underlying disease | Time lapse between treatment and cutaneous symptom (mo) | Variant type of cutaneous sarcoidosis | Outcome (mo) |
|---|---|---|---|---|---|---|---|
| 1 | Infliximab | Dubosc et al.3 | 54/F | SpA | 14 | Subcutaneous | Resolved (5) |
| 2 | Daïen et al.4 | 54/F | AS | 8 | Subcutaneous | Resolved (5) | |
| 3 | Clementine et al.5 | 70/M | AS | 60 | Erythema nodosum | Resolved (9) | |
| 4 | Dhaille et al.6 | 47/F | PsA | 4 | Erythema nodosum | Resolved (8) | |
| 5 | Takahashi et al.7 | 35/M | Crohn’s disease | 7 | Plaque | No improvement with topical corticosteroid | |
| 6 | Adalimumab | Daïen et al.4 | 53/F | AS | 21 | Erythema nodosum, subcutaneous | Resolved (3) |
| 7 | Dhaille et al.6 | 56/F | JRA | 1 | Subcutaneous, scar | Resolved (2) | |
| 8 | Lee et al.8 | 48/F | RA | 5 | Papular | Resolved (1) | |
| 9 | Santos et al.9 | 50/F | Sarcoidosis | 1 | Plaque | Resolved (not mentioned) | |
| 10 | Au et al.10 | 49/F | Sarcoidosis | 8 | Subcutaneous | Resolved (3) | |
| 11 | Etarnercept | Peno-Green et al.11 | 50/F | RA | 2 | NA | Resolved (1) |
| 12 | Hashkes and Shajrawi12 | 7/M | JA | 1 | Papular | Resolved (1.5) | |
| 13 | González-López et al.13 | 70/M | AS | 21 | Subcutaneous | Resolved (2) | |
| 14 | Verschueren et al.14 | 46/F | RA | 12 | Erythema nodosum | Resolved (1) | |
| 15 | Bachmeyer et al.15 | 39/M | AS | 1 | Papular | Unknown | |
| 16 | Daïen et al.4 | 46/M | PsA | 2 | Subcutaneous | Resolved (6) | |
| 17 | 72/F | RA | 18 | Scar | Resolved (1.5) | ||
| 18 | 69/F | RA | 27 | Erythema nodosum | Relapse (22) | ||
| 19 | Clementine et al.5 | 56/F | RA | 24 | Erythema nodosum, papular | Resolved (1.5) | |
| 20 | Lamrock and Brown16 | 56/F | PsA | 10 | Subcutaneous | Partially resolved (9) | |
| 21 | Burns et al.17 | 59/F | RA | 48 | Subcutaneous | Resolved (6) | |
| 22 | Unterstell et al.18 | 40/F | RA | 6 | Erythema nodosum | Resolved (6) | |
| 23 | Chaowattanapanit et al.19 | 50/M | SpA | 3 | Plaque | Resolved (3) | |
| 24 | Present case | 6/F | JRA | 14 | Erythrodermic | Resolved (5) |
References
1. Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009; 61:486–504.

2. Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. Extracutaneous disease. J Am Acad Dermatol. 2012; 66:719.e1–719.e10.
3. Dubosc AE, Perroud AM, Bagot M, Farenq V, Chevalier X, Maitre B, et al. Cutaneous granulomas during infliximab therapy for spondyloarthropathy. J Rheumatol. 2008; 35:1222–1223.
4. Daïen CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford). 2009; 48:883–886.

5. Clementine RR, Lyman J, Zakem J, Mallepalli J, Lindsey S, Quinet R. Tumor necrosis factor-alpha antagonist-induced sarcoidosis. J Clin Rheumatol. 2010; 16:274–279.

6. Dhaille F, Viseux V, Caudron A, Dadban A, Tribout C, Boumier P, et al. Cutaneous sarcoidosis occurring during anti-TNF-alpha treatment: report of two cases. Dermatology. 2010; 220:234–237.

7. Takahashi H, Kaneta K, Honma M, Ishida-Yamamoto A, Ashida T, Kohgo Y, et al. Sarcoidosis during infliximab therapy for Crohn's disease. J Dermatol. 2010; 37:471–474.

8. Lee SH, Kim SI, Song JS, Kim TH, Sohn JW, Kim SH, et al. Sarcoidosis induced by adalimumab in rheumatoid arthritis. Tuberc Respir Dis. 2011; 71:464–469.

9. Santos G, Sousa LE, João AM. Exacerbation of recalcitrant cutaneous sarcoidosis with adalimumab--a paradoxical effect? A case report. An Bras Dermatol. 2013; 88:6 Suppl 1. 26–28.

10. Au S, Mirsaeidi M, Aronson IK, Sweiss NJ. Adalimumab induced subcutaneous nodular sarcoidosis; a rare side effect of tumor necrosis factor-α inhibitor. Sarcoidosis Vasc Diffuse Lung Dis. 2014; 31:249–251.
11. Peno-Green L, Lluberas G, Kingsley T, Brantley S. Lung injury linked to etanercept therapy. Chest. 2002; 122:1858–1860.

12. Hashkes PJ, Shajrawi I. Sarcoid-related uveitis occurring during etanercept therapy. Clin Exp Rheumatol. 2003; 21:645–646.
13. González-López MA, Blanco R, González-Vela MC, Fernández-Llaca H, Rodríguez-Valverde V. Development of sarcoidosis during etanercept therapy. Arthritis Rheum. 2006; 55:817–820.

14. Verschueren K, Van Essche E, Verschueren P, Taelman V, Westhovens R. Development of sarcoidosis in etanercepttreated rheumatoid arthritis patients. Clin Rheumatol. 2007; 26:1969–1971.

15. Bachmeyer C, Blum L, Petitjean B, Kemiche F, Pertuiset E. Granulomatous tattoo reaction in a patient treated with etanercept. J Eur Acad Dermatol Venereol. 2007; 21:550–552.

16. Lamrock E, Brown P. Development of cutaneous sarcoidosis during treatment with tumour necrosis alpha factor antagonists. Australas J Dermatol. 2012; 53:e87–e90.

17. Burns AM, Green PJ, Pasternak S. Etanercept-induced cutaneous and pulmonary sarcoid-like granulomas resolving with adalimumab. J Cutan Pathol. 2012; 39:289–293.

18. Unterstell N, Bressan AL, Serpa LA, Fonseca e Castro PP, Gripp AC. Systemic sarcoidosis induced by etanercept: first Brazilian case report. An Bras Dermatol. 2013; 88:6 Suppl 1. 197–199.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download