Abstract
Background
Kinetin is a plant hormone that regulates growth and differentiation. Keratinocytes, the basic building blocks of the epidermis, function in maintaining the skin barrier.
Methods
To evaluate the efficacy of kinetin at the cellular level, expression of keratinocyte differentiation markers was assessed. Moreover, we examined the clinical efficacy of kinetin by evaluating skin moisture, transepidermal water loss (TEWL), and skin surface roughness in patients who used kinetin-containing cream. We performed quantitative real-time polymerase chain reaction to measure the expression of keratinocyte differentiation markers in HaCaT cells following treatment. A clinical trial was performed to assess skin moisture, TEWL, and evenness of skin texture in subjects who used kinetin-containing cream for 4 weeks.
Results
Kinetin increased involucrin, and keratin 1 mRNA in HaCaT cells. Moreover, use of a kinetin-containing cream improved skin moisture and TEWL while decreasing roughness of skin texture.
Conclusion
Kinetin induced the expression of keratinocyte differentiation markers, suggesting that it may affect differentiation to improve skin moisture content, TEWL, and other signs of skin aging. Therefore, kinetin is a potential new component for use in cosmetics as an anti-aging agent that improves the barrier function of skin.
The effects of human aging are mostly visible in the skin in such forms as increased wrinkling and sagging, as well as decreased elasticity1. Aging is also associated with physical disorders of the skin because its barrier function is disrupted, leading to a dry appearance and enhanced risk of skin disorders23. Understanding the mechanisms of skin aging is important for developing skin care products that delay aging and reduce damage45. Skin aging is induced by both intrinsic and extrinsic factors46 that lead to a reduction of structural integrity and loss of physiological function6.
Kinetin, a cytokine isolated in 1955, is an essential plant growth hormone that regulates cell growth and differentiation78. Kinetin has also been reported to be present in human cell extracts9 and urine10, and has been identified as a naturally occurring base modification agent of DNA11. Kinetin has been reported to have multiple functions, including anti-aging effects in cultured cells1213 and fruit flies14, antioxidant properties1516, antithrombotic activity1718, and cell differentiation effects192021. Therefore, in this study, we demonstrate that kinetin may induce anti-aging effects in skin by improving its barrier function.
HaCaT human keratinocytes (American Type Culture Collection, Boulecard Manassas, VA, USA) were cultured in Dulbecco's modified Eagle medium (Gibco/Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin (Gibco/Life Technologies) at 37℃ in an atmosphere of 5% CO2. Kinetin was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide.
HaCaT cells were seeded at a density of 3×103 cells in 96-well plates and incubated for 24 h. The cells were then incubated with kinetin (0~600 µM) for 24 h. HaCaT cell toxicity due to kinetin was evaluated using a water-soluble tetrazolium salt (WST)-1 assay (EZ-Cytox Cell Viability Assay Kit; Itsbio, Seoul, Korea). WST-1 solution was added to cultured cells at a volume equal to 10% that of the culture medium, and then the cells were incubated at 37℃ for 1 h. Cell viability was evaluated by measuring the absorbance at 450 nm using an iMark microplate reader (Bio-Rad, Hercules, CA, USA).
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. The purity and concentration of the RNA were evaluated using a MaestroNano®, a microvolume spectrophotometer (Maestrogen, Las Vegas, NV, USA). The recommended parameters of RNA quality for cDNA synthesis were OD 260/230 >1.8 and an OD 260/280 ratio within the range of 1.8~2.0. cDNAs were synthesized using the miScript II RT Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. To evaluate the expression of INV (forward primer: 5′-GGGTGGTTATTTATGTTTGGGTGG-3′, reverse primer: 5′-GCCAGGTCCAAGACATTCAAC-3′) and KRT1 (forward primer: 5′-ATTTCTGAGCTGAATCGTGTGATC-3′, reverse primer: 5′-CTTGGCATCCTTGAGGGCATT-3′), quantitative real-time polymerase chain reaction was performed using EvaGreen dye (Solis BioDyne, Tartu, Estonia) with Line-Gene K software (Bioer Technology Co., Ltd., Hangzhou, China). The CT value for each gene was normalized to β-actin (forward primer: 5′-GGATTCCTATGTGGGCGACGA-3′, reverse primer: 5′-CGCTCGGTGAGGATCTTCATG-3′). Relative expression levels of each gene were calculated using the 2−ΔΔCt method22.
The study protocols were approved by the Institutional Review Board of KISCS Incorporated (Cheongju, Korea) (IRB no. 1-70005239-A-N-01-2013-KISCS-ACA001-KSH). All subjects were informed about the objective of the study and provided informed consent and agreed to use products for skin care during the study. Forty women greater than 40 years of age were enrolled in a randomized, double-blind clinical trial (control group: 46.80±4.83 years, experiment group: 46.70±0.83 years). The subjects were selected based on age, the absence of skin conditions other than age-related conditions, and were not pregnant or nursing. All subjects were informed about the objective of the study, provided signed informed consent, and agreed to use only products for skin care during the study. Reasons for dropping out were itching, erythema, or excessive drinking or smoking. Subjects were divided into control and experimental groups consisting of 20 subjects each. All conditions were identical, other than the exposure of the experimental group to the test material. The study lasted four weeks, except no drop out. Clinical parameters were evaluated three times, namely, before application, and after 2 and 4 weeks of use. The investigator asked subjects about the condition of their skin and performed a visual examination of their skin condition, such as erythema, itching, scale, edema, tingling, and burning sensation, at every visit. The cream was prepared by incorporating the ingredients in the three phases (A, B, C). Ingredients in the A phase (distilled water, glycerin, 1,3-butylene glycol) were combined and heated until all the components were melted, and ingredients in the B phase (distilled water, dipotassium phosphate, sodium hydroxylate, kinetin) were combined and heated to the same temperature, to ensure homogeneity. The A and B phases were combined and emulsified using a homo mixer (Tokushu Kika Kogyo Co., Ltd., Osaka, Japan) at 5,000 rpm for 10 min. The mixture was cooled to 60℃ and blended with the homogenized phase C (emulium delta, sepipuls 400) at 5,000 rpm for 10 min. By then, the temperature of the mixture had dropped to 45℃. The mixture was combined and homogenized, while maintaining the pH at 6.2. The cream provided to the experimental group contained 2% (wt%) kinetin and the cream provided to the control group was prepared using the same volume of water in place of kinetin.
To investigate the improvement in skin barrier function, subjects were instructed to apply 2 g of test material to the face every morning and night for 4 weeks. Subjects and investigators were blinded to test and control treatments. Moisture and transepidermal water loss (TEWL) were measured on the right cheek and skin texture was evaluated on the left side of the forehead. At every visit, before measurements were taken, all subjects washed with the cleanser provided and lay quietly in a room with constant temperature (22℃±1℃) and humidity (45%±5%) so that all subjects would be evaluated under the same conditions.
To evaluate improvement in skin moisture, the DermaLab USB moisture probe (Cortex Technology Inc., Hadsund, Denmark) was applied and data were analyzed using the associated application software version 1.09. All subjects were measured on the same region of the right cheek five consecutive times, and the mean, maximum, and minimum values were determined. Measurements were taken three times, namely, before application, and after 2 and 4 weeks of use. The device applies the conductance measurement principle to measure the water-binding capacity of the stratum corneum (SC). This value correlates with skin moisture and is expressed in microsiemens (µS).
To evaluate improvement in TEWL, the DermaLab USB TEWL probe (Cortex Technology Inc.) was applied and data were analyzed using the associated application software version 1.09. Five consecutive measurements were taken on the subject's right cheek, and the mean, maximum, and minimum values were determined. Measurements were taken three times, namely, before application and at 2 and 4 weeks after the application.
Facial skin evenness measured by PRIMOS Lite (field of view 45×30; GFMesstechnik GmbH, Teltow, Germany) was used to obtain clinical images. Captured images were analyzed using the associated imaging software, PRIMOS Lite version 5.6E. Three consecutive clinical images of the left side of the subject's forehead were captured. Facial skin roughness was assessed based on the Ra value, which is the average of all heights and depths relative to the reference plane. Measurements were taken three times, namely, before application, and at 2 and 4 weeks after the application. The Ra value is the most widely used parameter of facial skin roughness and is the arithmetic mean of the maximum values of all measurements.
In cellular efficacy tests, all results are presented as the mean percentage±standard deviation of three independent experiments. Differences with a p-value of less than 0.05 or 0.001, as determined by Student's t-test, were considered statistically significant. In clinical efficacy tests, statistical analyses were conducted using SPSS software (PASW Statistics ver. 17.0 for Windows; IBM Co., Armonk, NY, USA). Paired t-tests were performed in cases of repeated measurements on the same subject. In clinical test, all statically analysis was compared between 0 week and 2 or 4 weeks using paired t-test which analyze statically significant by a comparison of experimental measured value of each entity in 0 weeks and 2 or 4 weeks. To analyze subject questionnaires, the mean values, standard deviation, and percentage were used. The formula used to measure the percent change for each skin parameter was
where A is defined as the individual value of any parameter at the 2 and 4 week visits and B represents the zero hour of the assessed parameter.
To determine whether kinetin affects HaCaT cell viability, cells were exposed to kinetin at concentrations ranging from 0~600 µM for 24 h. As shown in Fig. 1, kinetin reduced cell viability by 8.88% at 200 µM, 18.99% at 400 µM, and 29.78% at 600 µM. Kinetin-induced cytotoxicity increased significantly at concentrations greater than 200 µM. Thus, we used 200 µM as the maximum concentration in subsequent experiments.
Expression of involucrin, a marker of keratinocyte differentiation, was not altered by the treatment of HaCaT cells with 0.25 mM CaCl2. But when cells were treated with kinetin, involucrin mRNA increased in a dose-dependent manner. The addition of kinetin following treatment with 0.25 mM CaCl2 increased the level of involucrin mRNA to that of cells treated with 2 mM CaCl2 alone (Fig. 2). Simultaneously, we investigated the gene expression of another keratinocyte differentiation maker, keratin 1. As seen for involucrin, keratin 1 expression was not altered by treatment of HaCaT cells with 0.25 mM CaCl2. However, when these cells were treated with kinetin, keratin 1 expression increased markedly (Fig. 3).
Keratinocyte moisture content is pivotal for maintaining moisture in the skin. Normal keratinocytes maintain 10%~30% moisture; however, when moisture content drops below 10%, keratinocytes are unable to maintain the skin's barrier function as skin becomes dry, acquires an uneven texture, and produces wrinkles, which accelerate senescence23. In this study, to evaluate the efficacy of kinetin treatment on skin, we investigated the improvement in skin moisture after the treatment with a kinetin-containing cream.
We analyzed skin moisture content using the DermaLab USB moisture probe. Our data demonstrate that the moisture content of the control group, which used the non-kinetin-containing cream, was 388.10 µS before use, 389.96 µS after 2 weeks, and 390.72 µS after 4 weeks of application (Fig. 4). To compare the improvement at 2 and 4 weeks, we calculated and expressed the degree of improvement (Fig. 4). Consequently, the moisture content of the control group increased by 0.48% and 0.68% after 2 and 4 weeks, respectively. These changes were not statistically significant (p>0.05), indicating that the non-kinetin-containing cream had no measurable effect on moisture content. In contrast, the moisture content in the experimental group, which used kinetin-containing cream, was 413.34 µS before use, 551.39 µS after 2 weeks, and 619.98 µS after 4 weeks (Fig. 4). Interestingly, the use of the kinetin-containing cream significantly improved skin moisture by 33.40% and 49.99% after 2 and 4 weeks, respectively (p<0.001). These experiments demonstrate that the use of the kinetin-containing cream resulted in improved skin moisture content.
Next, to determine the efficacy of kinetin as a skin moisturizer, we used the DermaLab USB TEWL probe to investigate TEWL in the skin of subjects who had used control or kinetin-containing cream. The TEWL of control subjects was 9.59 g m−2 h−1 before use, 9.48 g m−2 h−1 after 2 weeks, and 9.38 g m−2 h−1 after 4 weeks (Fig. 5). To compare the improvement at 2 and 4 weeks, we calculated the improvement as a percentage based on the value before application. Consequently, the TEWL in the control group increased by 1.15% and 2.19% after 2 and 4 weeks of use, respectively. These changes were not statistically significant (p>0.05), indicating that the non-kinetin-containing cream had no measurable effect on TEWL. In contrast, TEWL in the experimental group was 9.96 g m−2 h−1 before use, 8.92 g m−2 h−1 after 2 weeks, and 7.08 g m−2 h−1 after 4 weeks (Fig. 5). To compare the improvement at 2 and 4 weeks, we calculated the improvement in TEWL as a percentage based on the value before application. Use of the kinetin-containing cream significantly improved TEWL by 10.40% and 28.88% after 2 and 4 weeks, respectively (p<0.001). Through these experiments, we identified the improvement in TEWL as an outcome of using kinetin-containing cream.
The thickness of the SC changes depending on its moisture content. Insufficient moisture in this layer roughens skin texture gradually24. Therefore, we investigated the efficacy of kinetin-containing cream on skin texture. Facial skin evenness was measured using PRIMOS Lite. Evenness in the control group was 20.10 Ra before use, 19.95 Ra after 2 weeks, and 19.79 Ra after 4 weeks (Fig. 6). To compare the improvement at 2 and 4 weeks, we calculated the improvement as a percentage based on the value before application. Consequently, skin texture in the control group improved by 0.77% and 1.54%, after 2 and 4 weeks of use, respectively. These data were not statistically significant (p>0.05), indicating that the non-kinetin-containing cream had no measurable effect on improving skin texture. In contrast, skin evenness in the experimental group was 20.84 Ra before use, 19.30 Ra after 2 weeks, and 16.77 Ra after 4 weeks (Fig. 6). To compare the improvement after 2 and 4 weeks, we calculated the improvement as a percentage based on the value before application. Using the kinetin-containing cream significantly improved the evenness of skin texture by 7.39% and 19.54% after 2 and 4 weeks (p<0.001), respectively. Through these experiments, we revealed that use of kinetin-containing cream can improve skin texture.
In this study, investigators asked the subjects individually about the condition of their skin and performed a visual evaluation of skin reactions, such as erythema, itching, scaling, tingling, tightness, prickling, and burning sensation at every visit. No extraordinary reactions were reported based on either visual evaluation or the questionnaire.
Kinetin has been reported to confer antioxidant and anti-aging effects in cultured cells12131516. However, whether kinetin plays a role in the barrier function of the skin remains to be investigated. Kinetin contributes to the delay in the skin aging process1319. In the present study, we examined whether kinetin reduces the aging process by improving skin barrier function. First, we evaluated the cytotoxicity of kinetin. HaCaT cell viability was affected at kinetin concentrations greater than 200 µM. Thus, 200 µM was used as the maximum concentration in subsequent experiments (Fig. 1). Our data demonstrate that the expression of keratinocyte differentiation markers, involucrin and keratin 1, was induced by kinetin in a dose-dependent manner (Fig. 2, 3). Furthermore, the expression of the major transcription factor p63 was upregulated by kinetin treatment (data not shown). Skin generates epidermal barrier by keratinocyte differentiation, which prevents water loss and maintain moisture25.
To evaluate the clinical efficacy of kinetin, we prepared a kinetin-containing cream and tested it on human skin. Because TEWL is used for assessing the epidermal barrier function, we used it as one of the parameters for evaluating the anti-aging effect of kinetin25 in a randomized, double-blind clinical trial. The control and experimental groups (n=20 each) used non-kinetin-containing and kinetin-containing creams, respectively. The skin moisture content and TEWL improved following the use of the kinetin-containing cream (Fig. 4, 5). Taken together, our results indicate that kinetin induced the formation of the skin barrier by accelerating keratinocyte differentiation.
Human skin acts as a barrier between the internal and external environment. Therefore, the skin protects the body from mechanical damage, noxious substances, and penetration by pathogens and radiation. The skin also plays a vital role in regulating body homeostasis by reducing TEWL to a minimum via the SC. Dysfunctional keratinocyte differentiation, such as in aging skin, leads to the thinning of the epidermis and destruction of the skin barrier, which can be caused by a reduction in filaggrin, a natural moisturizing factor synthesized by differentiating keratinocytes26. In addition, topical application of kinetin reduced spots, pores, and wrinkles, as well as improved the evenness of the skin texture27. Evenness of skin texture increased with the use of the kinetin-containing cream in a time-dependent manner (Fig. 6). Compared to pre-application roughness, evenness of the skin texture increased by 7.39% after 2 weeks of application and by 19.53% after 4 weeks (Fig. 6). Moreover, kinetin-containing cream was effective in treating wrinkles around the eye, such as crow's feet or under-eye wrinkles. In fact, using the kinetin-containing cream for 4 weeks reduced the number of under-eye wrinkles and the length and area of crow's feet (data not shown). These data demonstrate the clinical efficacy of kinetin on wrinkle elimination.
In conclusion, we identified a potential new cosmetic component derived from nature. We demonstrate the efficacy of kinetin for moisturizing and reducing the aging effects in both human keratinocytes in vitro and in the clinic. These findings indicate that kinetin reversed the effects of skin aging by modulating the skin barrier functions, suggesting that kinetin is a potentially useful component for various cosmetic uses.
Figures and Tables
 | Fig. 1Cytotoxicity of kinetin in HaCaT keratinocytes. HaCaT keratinocytes were treated with kinetin at the indicated concentrations for 24 h. The results are representative of three independent experiments (mean±standard deviation are shown). *p<0.05 and **p<0.001 as determined by the Student's t-test. |
 | Fig. 2The effect of kinetin on involucrin mRNA expression in HaCaT keratinocytes. Relative expression level of involucrin mRNA in CaCl2-treated (A) and CaCl2 plus kinetin-treated (B) HaCaT keratinocytes was determined by quantitative real-time polymerase chain reaction. The results are representative of three independent experiments (means±standard deviation are shown). |
 | Fig. 3The effect of kinetin on keratin 1 mRNA expression in HaCaT keratinocytes. Relative expression level of keratin 1 mRNA in CaCl2-treated (A) and CaCl2 plus kinetin-treated HaCaT keratinocytes was determined by quantitative real-time polymerase chain reaction. The results are representative of three independent experiments (means±standard deviation are shown). |
 | Fig. 4Improvement in skin moisture over time. Measurements were taken three times, namely before application and after 2 and 4 weeks of application. To evaluate improvement in skin moisture, DermaLab USB moisture probe was applied and data were analyzed using the associated software application version 1.09. *p<0.001 as determined by the Student's t-test. |
 | Fig. 5Percentage of improvement in transepidermal water loss (TEWL). Measurements were taken three times, namely, before application and after 2 and 4 weeks of use. *p<0.01 and **p< 0.001 as determined by the Student's t-test. |
 | Fig. 6Percentage of improvement in rates of skin roughness. Evenness of the skin surface was measured by PRIMOS Lite. Measurements were taken three times, namely, before application, and after 2 and 4 weeks of use. The captured images were analyzed using the associated imaging software PRIMOS Lite version 5.6E. *p<0.001 as determined by the Student's t-test. |
References
2. Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011; 29:3–14.

4. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008; 30:87–95.

5. Elsner P, Fluhr JW, Gehring W, Kerscher MJ, Krutmann J, Lademann J, et al. Anti-aging data and support claims--consensus statement. J Dtsch Dermatol Ges. 2011; 9:Suppl 3. S1–S32.
7. Miller CO, Skoog F, von Saltza MH, Strong FM. Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 1955; 77:1392.
8. Amasino R. 1955: kinetin arrives: the 50th anniversary of a new plant hormone. Plant Physiol. 2005; 138:1177–1184.

9. Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI. Evidence for the presence of kinetin in DNA and cell extracts. FEBS Lett. 1996; 393:197–200.

10. Barciszewski J, Mielcarek M, Stobiecki M, Siboska G, Clark BF. Identification of 6-furfuryladenine (kinetin) in human urine. Biochem Biophys Res Commun. 2000; 279:69–73.

11. Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI. Furfural, a precursor of the cytokinin hormone kinetin, and base propenals are formed by hydroxyl radical damage of DNA. Biochem Biophys Res Commun. 1997; 238:317–319.

12. Rattan SI, Clark BF. Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem Biophys Res Commun. 1994; 201:665–672.
13. Sharma SP, Kaur P, Rattan SI. Plant growth hormone kinetin delays ageing, prolongs the lifespan and slows down development of the fruitfly Zaprionus paravittiger. Biochem Biophys Res Commun. 1995; 216:1067–1071.

14. Sharma SP, Kaur J, Rattan SI. Increased longevity of kinetin-fed Zaprionus fruitflies is accompanied by their reduced fecundity and enhanced catalase activity. Biochem Mol Biol Int. 1997; 41:869–875.

15. Olsen A, Siboska GE, Clark BF, Rattan SI. N(6)-Furfury-ladenine, kinetin, protects against Fenton reaction-mediated oxidative damage to DNA. Biochem Biophys Res Commun. 1999; 265:499–502.

16. Verbeke P, Siboska GE, Clark BF, Rattan SI. Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Commun. 2000; 276:1265–1270.

17. Hsiao G, Shen MY, Lin KH, Chou CY, Tzu NH, Lin CH, et al. Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur J Pharmacol. 2003; 465:281–287.

18. Sheu JR, Hsiao G, Shen MY, Chou CY, Lin CH, Chen TF, et al. Inhibitory mechanisms of kinetin, a plant growthpromoting hormone, in platelet aggregation. Platelets. 2003; 14:189–196.

19. Lee JH, Chung KY, Bang D, Lee KH. Searching for aging-related proteins in human dermal microvascular endothelial cells treated with anti-aging agents. Proteomics. 2006; 6:1351–1361.

20. Ishii Y, Sakai S, Honma Y. Cytokinin-induced differentiation of human myeloid leukemia HL-60 cells is associated with the formation of nucleotides, but not with incorporation into DNA or RNA. Biochim Biophys Acta. 2003; 1643:11–24.

21. Berge U, Kristensen P, Rattan SI. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann N Y Acad Sci. 2006; 1067:332–336.

22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.

23. Muendnich K, Spann W, Reichenbach M, JACOBI . The clinical and forensic significance of complications after puncture of the maxillary sinuses. HNO. 1959; 8:24–28.
24. Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980; 75:500–507.

25. Wikramanayake TC, Stojadinovic O, Tomic-Canic M. Epidermal differentiation in barrier maintenance and wound healing. Adv Wound Care (New Rochelle). 2014; 3:272–280.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


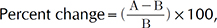
 XML Download
XML Download