Abstract
Glycolysis is a process that rapidly converts glucose to lactate to produce adenosine triphosphate (ATP) under anaerobic conditions and occurs in all eukaryotic and prokaryotic cells. On the other hand, the hexosamine biosynthetic pathway (HBP) converts glucose to intermediate products like UDP-N-acetylglucosamine, which is critical for post-translational modifications of proteins, such as protein glycosylation. These 2 pathways are well known to contribute to glucose metabolism, but recent studies indicate modulation of these pathways can alter immune system function. In this review article, the authors present results suggesting how cellular metabolism, including glycolysis and the HBP, occurs in immune cells, and the immunologic significances of such activities. In addition, they provide a review of the literature on the effects of glycolysis and the HBP on various autoimmune, immunologic, and allergic diseases. Finally, the authors briefly introduce the results of their research on the immunologic effects of HBP supplementation (glucosamine) in animal models of allergic disease.
Under aerobic conditions, cells with low metabolic demands (such as normal barrier cells, naïve T cells, and memory T cells) utilize oxidative phosphorylation to synthesize adenosine triphosphate (ATP), a robust fuel source. Oxidative phosphorylation is a highly efficient process that produces 36 to 38 ATP molecules from 1 molecule of glucose, but under anaerobic conditions, this process is precluded, and instead cells generate energy by glycolysis, which involves the conversion of glucose to lactate in order to synthesize ATP. Although glycolysis can synthesize ATP rapidly, it is much less efficient than oxidative phosphorylation, because oxidative phosphorylation generates 36 to 38, but glycolysis generates only 2 at times postoperatively.1
Recent studies indicate that cells with high metabolic demands, such as tumor cells and activated T cells, synthesize ATP through glycolysis during rapid proliferation, even under aerobic conditions. The first scientist to discover this phenomenon was Otto Warburg, who noted cancerous cells convert glucose to lactate in order to synthesize ATP even under oxygen-rich conditions, and thus “aerobic glycolysis” is also referred to as “the Warburg effect.”2
Aerobic glycolysis has been studied primarily in tumor cells, and attempts have been made to inhibit the growth and progression of neoplasia by regulating this process.34 However, studies on the role of aerobic glycolysis in immune cells are in their infancy.
The hexosamine biosynthetic pathway (HBP) is also involved in the metabolism of glucose. The HBP causes post-translational modifications of proteins and contributes to syntheses of complex molecules, such as glycolipids, proteoglycans, and others. Furthermore, recent studies have shown that a competitive relationship exists between the HBP and glycolysis.567
Indeed, “metabolic immunology” or “immunometabolism,” that is, a study of the role of metabolism on immunologic functions and activities of cells, has recently attracted much attention in a hitherto relatively unexplored field of research. Thus, in this review article, we first describe how cellular metabolic pathways, including glycolysis and HBP, appear to act within cells of the immune system and comment on the immunological significances of these activities. Next, we review the effects of glycolysis and the HBP on various autoimmune diseases and immunological/allergic diseases. Finally, we briefly introduce the results of our research on the immune effects of HBP augmentation in animal models of allergic disease.
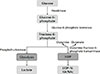
A schematic of glycolysis and the HBP is provided in Figure. Importantly, the final product of glycolysis is lactate, whereas that of the HBP is UDP-N-acetylglucosamine (UDP-N-GlcNAc). The purposes of these pathways also differ from each other. Glycolysis is used to rapidly synthesize ATP, whereas the HBP is used to post-translationally modify numerous proteins. Glucosamine (GlcN) administration has the effect of increasing the availability of substrate required for HBP activation, and the final product of the HBP is UDP-N-GlcNAc, which is subsequently converted to O-GlcNAc by O-GlcNAc transferase. Furthermore, it has been well established that O-GlcNAc levels increases sharply under stressful conditions, at least in a short term, and that these increases have cyto-protective effects.89 It has also been shown in a contrast-induced acute kidney injury rat model that when O-GlcNAc signaling is enhanced by GlcN administration, oxidative stress and apoptosis are reduced.10
Normal T cells have frequently been used to study the role of aerobic glycolysis, as they proliferate and become activated when exposed to external stimuli, such as extracellular antigens or pathogens. Furthermore, when naïve T cells are activated, aerobic glycolysis is required to regulate the preferential translation of interferon gamma (IFN-γ) mRNA and optimize IFN-γ secretion.11 Aerobic glycolysis also increases the anti-tumor activities of T cells and promotes the differentiation of naïve T cells into Th17 cells, rather than regulatory T (Treg) cells.121314
Goto et al.15 found that procyanidin, a type of flavonoid found mainly in fruits like apples, inhibits glycolysis. Interestingly, this group reported that when proliferating CD4+ T cells were stimulated with anti-CD3ε monoclonal antibody in the presence of procyanidin, which was administered to inhibit glycolysis, cellular proliferative activity was reduced to 10% of its normal level and the levels of IFN-γ, interleukin (IL)-4, IL-6, and IL-10 were also significantly reduced.
HBP synthesizes UDP-N-GlcNAc from glucose, and UDP-N-GlcNAc then becomes a substrate for glycoprotein synthesis, through N-glycan synthesis.161718 Araujo et al.1 showed that when T cells are treated with GlcNAc, it not only significantly inhibits Th17 differentiation, but also promotes differentiation into Treg cells.
N-glycan branching reduces T-cell receptor clustering/signaling and inhibits T-cell growth by increasing the surface retention of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), an inhibitor of T-cell growth.11920 However, inhibition of aerobic glycolysis allows fructose-6-phosphate to be used as a substrate for the HBP, which results in increased N-glycan branching.1
Both aerobic glycolysis and the HBP require fructose-6-phosphate as an intermediate, and thus these 2 pathways have a competitive relationship, that is, if one pathway is utilized, shunting toward the other pathway may be inhibited. However, Araujo et al.1 observed that extracellular acidification and oxygen consumption rates did not change significantly even when GlcNAc was administered to T cells cultured under conditions that induce a Th17 phenotype, and suggested that GlcNAc administration did not directly inhibit aerobic glycolysis in their system.
Garcia-Carbonell et al.21 extracted fibroblast-like synoviocytes from the joint fluids of rheumatoid arthritis patients and treated these primary cell lines with 2-deoxy-D-glucose (2-DG), an inhibitor of glycolysis. Cellular proliferation and migration rates were significantly diminished by 2-DG, which also significantly inhibited secretions of IL-6, matrix metalloproteinase (MMP)-1, and MMP-3. To confirm these results, additional experiments were conducted in animal models. In a K/BxN murine serum transfer model of arthritis, administration of a glycolysis inhibitor (2-DG) significantly reduced inflammatory cell infiltration, joint injury severity, and cartilage damage. Collectively, these findings suggest that inhibition of glycolysis pathways offers the possibility of an adjunctive strategy for the treatment of rheumatoid arthritis.
In T-cell activation assays, inhibition of T-cell receptor signaling and proliferation has been demonstrated in naïve T cells cotreated with hexosamine and GlcNAc or GlcN.14 Furthermore, hexosamine supplementation in mice with experimental autoimmune encephalomyelitis accelerated disease progression.17
Salvatore et al.22 administered GlcNAc orally or trans-rectally to pediatric patients with refractory inflammatory bowel disease (Crohn's disease or ulcerative colitis). Eight of the 12 (8/12) patients administered GlcNAc exhibited significant improvement, and 5 of the 9 patients administered GlcNAc trans-rectally) reported marked improvement (2 reported complete remission and 3 notable improvements in symptoms). All 21 patients underwent mucosal biopsy and were histologically confirmed to have lower gastrointestinal disease improvements. The authors concluded GlcNAc offers an affordable, non-toxic, and effective treatment for refractory inflammatory bowel disease.
Attempts have been made to develop a targeted drug delivery system based on the sequential, selective inhibition of each step of aerobic glycolysis (Figure).23 Pharmacologic agents, such as phloretin, WZB117, and fasentin, inhibit glycolysis by preventing cellular glucose uptake through glucose transporter (GLUT), but as GLUTs are present in all cells, blockade of GLUTs in specific cell types has proven to be difficult. Therefore, this treatment type remains at the pre-clinical stage.2425
Hexokinase coverts glucose to glucose-6-phosphate in the first step of glycolysis. Lonidamine blocks hexokinase and has passed phase III clinical trials, but has not been widely commercialized due to its reported pancreatic and hepatic toxicity profiles.262728
The glucose analog 2-deoxyglucose also potently inhibits glycolysis, and although pre-clinical trials have been attempted, reports suggest that its effect is somewhat unpredictable and that its use may be associated with increased transformed cell survival.29303132
Agents that inhibit phosphofructokinase (PFK), which converts fructose-6-phosphate to fructose-1,6,-bisphosphate, are also being actively developed.33 In addition, a number of agents have been developed to inhibit enzymes involved in downstream steps of glycolysis, for example, 3-bromopyruvate is an inhibitor of glyceraldehyde-3-phosphate dehydrogenase (GAPDH),34353637 FX-11 and oxamate suppress lactate dehydrogenase (LDH),3839 and Shikonin and pyruvate kinase M2 (PKM2)-specific siRNA are inhibitors of pyruvate kinase (PK).4041 Since these potential therapeutics reached pre-clinical or early clinical stages, they may soon be available for use in clinical practice.
Jin et al.42 investigated the role of GlcNAc in systemic anaphylactic shock and ear swelling. Systemic anaphylaxis and ear swelling were induced by administering compound 48/80 (a mast cell degranulator) intraperitoneally in ICR mice. In order to evaluate its anti-allergic effects, GlcNAc was administered orally or subcutaneously 1 hour prior to administering compound 48/80. GlcNAc was found significantly reduce mortality and ear swelling.
Jin et al.43 performed a randomized, double-blinded, placebo-controlled, parallel clinical trial to evaluate the efficacy of low-dose cyclosporine and GlcN combination therapy in patients with atopic dermatitis (AD). Patients with AD and a severity scoring of atopic dermatitis (SCORAD) index of ≥30 (maximum score 103) were selected and cyclosporine (2 mg/kg) plus GlcN (25 mg/kg) was given to one group of patients, and cyclosporine (2 mg/kg) and placebo to another. After 8 weeks of drug administration, the mean SCORAD index of patients given cyclosporine plus GlcN was significantly greater, and no significant increase in side effects was observed. As a result, they recommended that low-dose cyclosporine plus GlcN be considered for the treatment of moderate to severe AD.
We sought to evaluate the effect of hexosamine supplementation on experimental allergic inflammation. Initially, we induced allergic asthma and rhinitis in BALB/c mice by intraperitoneal or intranasal administration of ovalbumin (an experimental protein derived from chicken egg white). In order to evaluate the therapeutic effect of GlcN supplementation, GlcN was administered intravenously to mice 30 minutes prior to each of the ovalbumin administrations. After 4 weeks of treatment, serum immunoglobulin G (IgE) levels were significantly lower in the in GlcN group than in the untreated asthma/rhinitis groups. On a cellular level, we noted that the numbers of inflammatory leukocytes, such as eosinophils, and Th1 and Th17 cytokine titers in bronchoalveolar lavage fluid were all significantly reduced by GlcN treatment. Histopathologic findings of lung parenchyma and nasal mucosa were also significantly better in treated mice. Taken together, these findings suggest that supplementary GlcN treatment may augment treatment response by up-regulating the HBP pathway.44
GlcN is an over-the-counter, non-prescription dietary supplement. However, because it is not classified as a drug, few studies have been conducted to determine its side effect profile and/or drug interactions.45 Adverse reactions induced by GlcN and chondroitin sulfate have been reported in fewer than 5% of patients. However, gastrointestinal side effects, such as discomfort, abdominal pain, diarrhea, and nausea, are relatively common, and somnolence, cutaneous reactions and headaches have also been reported.46
Cerda et al.46 asked 151 patients with chronic liver disease whether they had taken or were taking GlcN and/or chondroitin sulfate. Twenty-three patients (15.2%) had taken GlcN preparations, and 2 reported a temporary increase in liver enzyme (aminotransferase) levels after administration. The cause of rare hepatotoxicity attributable to GlcN has not been determined, but it is currently believed to be related to a hypersensitivity mechanism. Accordingly, caution should be exercised when GlcN is administered to patients with impaired hepatic function; and if elevated liver transaminase levels or jaundice appear, GlcN should be discontinued and a specialist consulted.
One case of transient asthma deterioration has been reported after GlcN dietary supplementation. However, the causal relationship between GlcN administration and asthma exacerbation is unclear and it is difficult to conclude from this single incident whether GlcN was involved. MEDLINE searches conducted for this review did not identify any other GlcN-associated respiratory complications.47
GlcN is extracted from the exoskeleton of crab, lobster, and shrimp, and thus patients with a seafood or shellfish allergy may be hesitant to take GlcN. However, this allergy is caused by IgE responses to antigens in seafood flesh and is not related with shells. Gray et al.48 conducted a GlcN skin test on 6 patients with allergies to seafood, such as shrimp, crab, and lobster, and all were negative for reaction to GlcN. After skin testing, these 6 patients were administered 500 mg of oral GlcN and no reported side effect or meaningful symptom was observed.
Finally, this review aimed to introduce some of the most recently updated articles on the possible pathophysiologic mechanism between glycolysis and immune function. First, Layman et al.49 have focused on a protein called neural precursor cell expressed, developmentally down-regulated 4 (Nedd4) family interacting protein 1 (Ndfip1). When this protein binds to the Nedd4 E3 ligase, it suppresses the Th2 immune response by decreasing the secretion of cytokines, such as IL-4 from the Th2 lymphocyte.5051 Layman et al.49 studied the relationship between this Ndfip1 protein and Treg cell. Treg cells in a normal stable state express Foxp3 protein and play a role in immune regulation. Treg lymphocytes therefore play a role in protecting against autoimmune diseases and inflammatory disorders.5253 However, when unstable in certain circumstances, Foxp3 protein expression is suppressed, while pro-inflammatory cytokine is secreted from activated Treg cells.5455 Layman et al.49 developed a knockout mice strain (Ndfip1fl/fl Foxp3-Cre mice) in which the Ndfip1 protein was specifically knocked out only in Treg cells. In these mice, severe inflammation, such as splenomegaly, lymphadenopathy, dermatitis, esophagitis, or pneumonia, spontaneously developed from 9 to 16 weeks of age. In the serum of these mice, immunoglobulins, such as IgE and immunoglobulin G1 (IgG1), were also significantly increased compared to wild type mice. The important point here is that glycolysis is significantly increased in Ndfip-1 deficient Treg cells. The glycolytic rate (measured as extracellular acidification rate [ECAR]) and glycolytic capacity were significantly increased in Ndfip-1-deficient Treg cells compared to wild-type Treg cells. Therefore, it is possible that the switching of metabolism into glycolysis in activated Treg cells is closely related to pathophysiology of Ndfip1 knockout-induced autoimmune disorders.
Cai et al.56 studied the role of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in nasopharyngeal cancer (NPC) patients. LMP1 is a kind of oncoprotein that plays an essential role in maintaining EBV in the latent infection state and activating angiogenesis. As a result, LMP1 promotes tumor cell invasion and, eventually, distant metastasis.5758 LMP1 is also known to contribute to the increase of myeloid-dependent suppressor cells (MDSCs).56 In this study, they examined the relationship between LMP1 activation and MDSC expansion and found that activation of glycolysis is involved as an important mechanism. They measured ECAR to identify glycolytic activity in NPC cells expressing LMP1. As a result, it was found that LMP1-expressing cells showed a marked increase in glycolytic activity (a significant increase in ECAR and abundant lactate as a degradation product). In addition, they suggested that gene expression of various enzymes related to glycolysis (GLUT-1, HK-2, GPI, and PFK) was significantly increased in NPC cells. On the other hand, in GLUT-1-knockout cells using GLUT-1 siRNA, the glycolytic pathway was markedly decreased and the expression of various genes related to glycolysis was significantly reduced. Therefore, researchers have suggested that increased GLUT-1 dependent glycolysis is closely related to malignant cell transformation and increased MDSCs.56
Glycolysis and the HBP are both associated with glucose metabolism and exhibit important and fascinating connections with the immune system. Notably, these 2 pathways play critical roles in the proliferation and activation of leukocytes, such as T cells, and exhibit a competitive relationship whereby the down-regulation of glycolysis upregulates the HBP. Although attempts to treat malignant tumors and immunological diseases using drugs that modulate these pathways are ongoing, further research is needed before these molecules can be safely and effectively used in clinical practice. GlcN appears to have a satisfactory safety profile and has been shown by our group to be effective in inhibiting the progressions of experimental inflammatory diseases, such as allergic asthma and rhinitis, in mice. Further studies are needed to expand their clinical applications.
Figures and Tables
References
1. Araujo L, Khim P, Mkhikian H, Mortales CL, Demetriou M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 2017; 6:e21330.

2. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927; 8:519–530.

3. Liu W, Zhang B, Hu Q, Qin Y, Xu W, Shi S, et al. A new facet of NDRG1 in pancreatic ductal adenocarcinoma: suppression of glycolytic metabolism. Int J Oncol. 2017; 50:1792–1800.

4. Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017; 36:44.

5. Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001; 291:2376–2378.

6. Vosseller K, Sakabe K, Wells L, Hart GW. Diverse regulation of protein function by O-GlcNAc: a nuclear and cytoplasmic carbohydrate post-translational modification. Curr Opin Chem Biol. 2002; 6:851–857.

7. Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004; 1673:13–28.

8. Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004; 279:30133–30142.
9. Chatham JC, Marchase RB. Protein O-GlcNAcylation: a critical regulator of the cellular response to stress. Curr Signal Transduct Ther. 2010; 5:49–59.

10. Hu J, Chen R, Jia P, Fang Y, Liu T, Song N, et al. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic Biol Med. 2017; 103:121–132.

11. Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O'Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013; 153:1239–1251.

12. Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015; 162:1217–1228.

13. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011; 186:3299–3303.

14. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011; 208:1367–1376.
15. Goto M, Wakagi M, Shoji T, Takano-Ishikawa Y. Oligomeric procyanidins interfere with glycolysis of activated T cells. A novel mechanism for inhibition of T cell function. Molecules. 2015; 20:19014–19026.

16. Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009; 139:1229–1241.

17. Grigorian A, Lee SU, Tian W, Chen IJ, Gao G, Mendelsohn R, et al. Control of T Cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem. 2007; 282:20027–20035.

18. Grigorian A, Araujo L, Naidu NN, Place DJ, Choudhury B, Demetriou M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J Biol Chem. 2011; 286:40133–40141.

19. Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001; 409:733–739.

20. Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007; 129:123–134.

21. Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2016; 68:1614–1626.

22. Salvatore S, Heuschkel R, Tomlin S, Davies SE, Edwards S, Walker-Smith JA, et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol Ther. 2000; 14:1567–1579.

23. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013; 12:152.

24. Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005; 202:654–662.

25. Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012; 11:1672–1682.

26. Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006; 25:4777–4786.

27. Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer's stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009; 19:17–24.

28. Price GS, Page RL, Riviere JE, Cline JM, Thrall DE. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol. 1996; 38:129–135.

29. Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004; 53:116–122.

30. Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M, et al. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther. 2007; 6:3049–3058.

31. Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008; 7:809–817.

32. Zhong D, Xiong L, Liu T, Liu X, Liu X, Chen J, et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem. 2009; 284:23225–23233.

33. Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008; 7:110–120.

34. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004; 324:269–275.

35. Pedersen PL. The cancer cell's “power plants” as promising therapeutic targets: an overview. J Bioenerg Biomembr. 2007; 39:1–12.

36. Ganapathy-Kanniappan S, Vali M, Kunjithapatham R, Buijs M, Syed LH, Rao PP, et al. 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol. 2010; 11:510–517.

37. Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF. Anticancer efficacy of the metabolic blocker 3-bromopyruvate: specific molecular targeting. Anticancer Res. 2013; 33:13–20.
38. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010; 107:2037–2042.

39. Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010; 9:33.

40. Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011; 30:4297–4306.

41. Goldberg MS, Sharp PA. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med. 2012; 209:217–224.

42. Jin SE, Jung J, Jun J, Jeon DW, Kim HM, Jeong HJ. Anti-allergic activity of crystallinity controlled N-acetyl glucosamine. Immunopharmacol Immunotoxicol. 2012; 34:991–1000.

43. Jin SY, Lim WS, Sung NH, Cheong KA, Lee AY. Combination of glucosamine and low-dose cyclosporine for atopic dermatitis treatment: a randomized, placebo-controlled, double-blind, parallel clinical trial. Dermatol Ther. 2015; 28:44–51.

44. Jung AY, Heo MJ, Kim YH. Glucosamine has an antiallergic effect in mice with allergic asthma and rhinitis. Int Forum Allergy Rhinol. 2017; 7:763–769.

46. Cerda C, Bruguera M, Parés A. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J Gastroenterol. 2013; 19:5381–5384.

47. Tallia AF, Cardone DA. Asthma exacerbation associated with glucosamine-chondroitin supplement. J Am Board Fam Pract. 2002; 15:481–484.
48. Gray HC, Hutcheson PS, Slavin RG. Is glucosamine safe in patients with seafood allergy? J Allergy Clin Immunol. 2004; 114:459–460.

49. Layman AAK, Deng G, O'Leary CE, Tadros S, Thomas RM, Dybas JM, et al. Ndfip1 restricts mTORC1 signalling and glycolysis in regulatory T cells to prevent autoinflammatory disease. Nat Commun. 2017; 8:15677.

50. Oliver PM, Cao X, Worthen GS, Shi P, Briones N, MacLeod M, et al. Ndfip1 protein promotes the function of itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity. 2006; 25:929–940.

51. Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol. 2002; 3:281–287.

52. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155:1151–1164.
53. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001; 27:68–73.

54. Overacre AE, Vignali DA. T(reg) stability: to be or not to be. Curr Opin Immunol. 2016; 39:39–43.

55. Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013; 13:461–467.

56. Cai TT, Ye SB, Liu YN, He J, Chen QY, Mai HQ, et al. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathog. 2017; 13:e1006503.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download