Abstract
Purpose
Chinese herbal medicine (CHM) has been widely used in China to treat allergic rhinitis (AR). However, several studies have produced conflicting data with regard to the efficacy of the medicine. Our aim was to perform a meta-analysis of randomized clinical trials (RCTs) to evaluate the relative efficacy of CHM.
Methods
We systematically searched the PubMed, Medline, and Springer electronic databases up to March 2017 for RCTs comparing the efficacy of CHM versus placebo for the treatment of patients with AR. Total nasal symptoms and quality of life were assessed through pooling mean difference (MD) with its 95% confidence interval (CI). Moreover, sensitivity and subgroup analyses according to control design and quality of life assessment were performed to evaluate the source of heterogeneity.
Results
Eleven RCTs were enrolled in the meta-analysis. Assessment of overall heterogeneity indicated significant heterogeneity among the individual studies (I2=100%, P<0.00001), and thus ransomed effects model was used to pool data. CHM was found to significantly enhance quality of life compared with placebo (MD=-0.88, (95% CI: -1.55, -0.21); P=0.01). The symptom of itchy nose, sneezing or total nasal symptoms scores were not significantly improved after CHM treatment, although the improvement in itchy nose just failed to reach significance (MD=0.09, (95% CI: 0.00, 0.18); P=0.06).
Conclusions
This study suggests that CHM appears to improve the quality of life of AR patients. However, these findings, as well as the findings for the effect of CHM on sneezing, total nasal symptoms, and the symptom of itchy nose, need to be substantiated in larger cohorts of AR patients by further well-designed studies.
Allergic rhinitis (AR) is an IgE-mediated disease,1 which shows a high prevalence and significantly affects the patient's quality of life. In China, the prevalence of AR increased among adults and children over the last decades, ranging from 8% to 24.1%. Meanwhile, the disease affects 6.2% and 7.2% of the adults in rural and urban areas, respectively.23
Although AR is not a life-threatening disease, it remains a significant health problem in China, because the main symptoms of sneezing, itching, runny nose, and nasal congestion are often associated with the impaired quality of life, sleep quality, and mental state of AR patients. Furthermore, AR imposes a great financial burden on both the individual and society due to health care and social costs associated with the disease.45 Conventional treatment of AR includes intranasal corticosteroids, antihistamines, decongestants, cromolyn, and leukotriene receptor antagonists.16 Epidemiologic evidence has indicated that AR is associated with the development of asthma and chronic rhinosinusitis,7 which add to the overall burden of the disease.
Chinese herbal medicine (CHM) has been widely used to treat AR for centuries; with the herbs resulting in AR symptom remission through immune modulation and anti-allergic or anti-inflammatory effects. Indeed, several clinical studies have evaluated the effects of CHM, such as Yu-ping-feng San (YS), Cure-allergic-rhinitis syrup (CS), fermented red ginseng, or Biminne capsules.89 Wang et al.10 have suggested that CHM therapy is useful for the treatment of nasal symptoms in AR patients, based on the effect of decreased nasal symptoms in patients enrolled in seven randomized controlled trials (RCTs). However, some clinical trials have subsequently provided conflicting data for the potential benefit of CHM for AR,1112 Itching and sneezing represent two of the main bothersome symptoms of AR, and activation of the central and peripheral nervous systems plays an important role in the 2 processes.13 Moreover, protective function of CHM has been proved.14 The purpose of this meta-analysis was therefore to further evaluate the potential efficacy of CHM for the treatment of AR symptoms, including total nasal symptom, itching, sneezing, and quality of life.
The study was performed in accordance with the recommendations of the Cochrane Collaboration and the PRISMA 2009 guidelines.
A systematic search was performed for randomized controlled trials (RCT) comparing the effect of CHM versus conventional western medicine on symptoms in patients with AR, using the PubMed, Medline, and Springer databases up to March 2017. The employed search terms were ‘Chinese Medicine’ or “herbal” or “eastern medicine” or alternative medicine” or “natural medicine” and “allergic rhinitis” or “AR.” Cited references of the studies included in the meta-analysis were also searched for clinical trials/studies, which may have been missed by the initial search.
Two reviewers independently identified studies for eligibility. Any disagreement on the suitability of a study for inclusion in the meta-analysis was resolved by discussion until reaching a general consensus.
Only studies published in English were included in the meta-analysis if they met the following criteria: (1) the study was designed as a randomized controlled trial; (2) patients had typical symptoms of AR, and elevated total blood IgE level or positive skin prick test reactions were observed; (3) patients were treated with traditional Chinese medicine as compared with placebo or conventional Western medicine; (4) One of the following outcomes was reported─sneezing, itchy nose, total nasal symptom score (TNSS), and quality of life measured by Rhino conjunctivitis Quality of Life (RQLQ) or 36-item Short Form Health Survey (SF-36); (5) patients had provided informed written consent prior to entry to the study.
Reviews, meetings abstracts, case reports, and comments were excluded from the meta-analysis.
Data extraction was performed independently by 2 researchers according to a predefined information sheet, which included details on patient characteristics (enrolled number, distribution area, and age), experimental and control intervention, and main outcome measures.
Quality of the included studies was assessed by 2 independent authors using the risk of bias tools based on the Cochrane Handbook version 5.1.0.15 Briefly, 6 bias items were assessed, such as selection bias, performance bias, detection bias, attrition bias, reporting bias, and others. Each item was categorized as low, high, or unclear risk.
The meta-analysis evaluating the effectiveness and safety of CHM in treating AR was performed using RevMan 5.2. Mean difference (MD) and 95% confidence intervals (CIs) were calculated to evaluate the differences on nasal symptom and quality of life after CHM treatment compared with control. Heterogeneity was assessed using the Cochrane Q and I2 statistics.1617 The Q test evaluates the contribution of each study by its inverse variance, which is computed by summing the squared deviations of each study's effect estimate from the overall effect estimate. Notably, the Q test only informs the analyst about the presence versus the absence of heterogeneity.16 The I2 index describes the percentage of total variation across studies that is due to heterogeneity rather than chance, and can be readily calculated from basic results obtained from a typical meta-analysis as I2=100%×(Q-df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.17
P value for heterogeneity <0.05 and/or I2>50% defined occurrence of significant heterogeneity. Bayesian methods were subsequently used to fit the random-effects meta-analysis models.18 The fixed-effect model was applied to pool effective data when insignificant heterogeneity was observed among individual studies. Subgroup analysis was stratified by control intervention as placebo or conventional Western medicine. Moreover, meta-analysis of the quality of life data was performed by subgroup analysis according to SF-36 or RQLQ. Publication bias was analyzed using Funnel plots, which are simple scatterplots of the treatment effects estimated from individual studies against a measure of study size.
Fig. 1 shows the process by which the studies were selected for inclusion in the meta-analysis. A total of 323 potential studies were screened initially, of which 106 duplicate studies were excluded and the remaining 217 articles were further evaluated for specific relevance to the meta-analysis. After further exclusion of 97 deemed to be irrelevant, the abstracts of the remaining 120 articles were reviewed in greater detail for specific information pertinent to the meta-analysis. Eighty four articles, including 36 nonrandomized controlled trails (non-RCTs), 28 non-Chinese herbal medicine trials, and 20 trials which did not provide detailed information on the outcomes assessed, were further excluded. Totally, 36 articles were fully reviewed in detail, and 25 articles were excluded (14 non-RCT; 9 could not extract data which just provided descriptive statistics; 2 by Xue et al.1920 were enrolled in the overlapped population and 1 study was enrolled; 1 study protocol which did not show study results). Finally, 12 trails documented in 11 articles were enrolled in the meta-analysis.89121921222324252627
The characteristics of the studies included are summarized in Table. Among the enrolled studies, 6 were conducted in China (2 studies each in mainland China, Hong Kong, and Taiwan). Six studies reported scores evaluating the quality of life, and 5 studies reported nasal symptom scores.
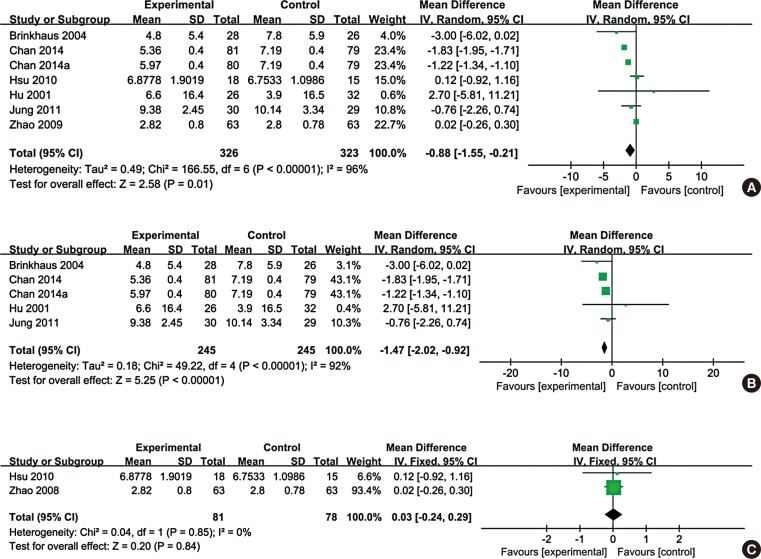
Fig. 2 shows an overview of the risk of bias. Six studies did not report any details about randomization and 6 trials were designed as double-blind indicating their overall eligibility for inclusion in the meta-analysis. All enrolled studies were at low risk of bias on allocation concealment. The studies by Min et al.12 and Chui et al.22 did not perform random sequence generation. Moreover, outcome assessment was not blinded in the study by Min et al.12 A high risk of bias due to confounding and missing data was also observed in 2 studies.1927 Overall, the enrolled studies were suitable for the meta-analysis with a moderate risk of bias.
Nasal symptoms, such as sneezing and itchy nose, were assessed. Four studies, including 283 patients treated by CHM and 266 patients in the control group, were evaluated for these symptoms. As shown in Fig. 3, no significant difference was found for sneezing (MD=0.02, 95% CI: -0.11, 0.15) in patients treated with CHM compared with patients treated with control medicine, and no significant heterogeneity among individual studies was observed (I2=0%, P=0.40). In contrast, significant improvement was found on itchy nose after CHM treatment as compared with controls (MD=0.09, 95% CI: 0.00, 0.18) and also no significant heterogeneity was found among the individual studies (I2=0%, P=0.76).
Data on total nasal symptoms, including sneezing, nasal discharge, nasal itch, and nasal obstruction, were reported in 6 trials in 5 studies (Fig. 4A). Briefly, 324 patients were treated with CHM and 298 patients with control medication. Assessment of overall heterogeneity indicated that there was significant heterogeneity among the individual studies (I2=100%, P<0.00001), and thus we used the ransomed effects model to pool data. Analysis of the data for total symptoms showed that CHM treatment did not lead to significant improvement in nasal symptoms in AR patients as compared with control medication (MD=-0.59, 95% CI: -1.33, 0.16).
Subgroup analysis was stratified by control intervention as placebo or Western medicine. As shown in Fig. 4B, 5 trails were designed as CHM versus placebo. There was no trend for decreasing significant heterogeneity (I2=100%, P<0.00001), and the ransomed effects model was used to pool data. Analysis of the data for total symptoms showed that CHM treatment did not lead to significant improvement in nasal symptoms in AR patients as compared with placebo (MD=-0.22, 95% CI: -1.01, 0.58).
In order to explore the stability of the meta-analysis, sensitive analysis was performed, and the result was not inversed after removal of each study at one time, indicating similar treatment efficacy of CHM versus control medication.
Total quality of life based on RQLQ or SF-36 was evaluated in 7 trials from 6 studies (Fig. 5A). Among the 6 studies, quality of life in the studies by Hsu et al.23 and Zhao et al.21 was assessed by SF-36. Patients in 5 other studies were all assessed for quality of life, but statistical data could not be extracted.
Overall, data from 326 patients treated with CHM and 323 patients treated with the control medication were evaluated in the meta-analysis. Assessment of overall heterogeneity indicated significant heterogeneity among the individual studies (I2=96%, P<0.00001), and thus the data were pooled using the ransomed effects model. Analysis of the pooled data indicated that CHM treatment significantly improved the quality of life for AR patients as compared with control medication (MD=-0.88, 95% CI: -1.55, -0.21).
Subgroup analysis was performed for data obtained with SF-36 or RQLQ. Fig. 5B shows that CHM treatment significantly improved RQLQ for AR patients compared with control medication (MD=-1.47, 95% CI: -2.02, -0.92). In contrast, based on SF-36 data, the observed heterogeneity was significantly reduced (I2=0%, P=0.85) and no significant difference was found in CHM improvement on SF-36 for AR patients, compared with control medication (MD=0.03, 95% CI: -0.24, 0.29).
CHM has been widely used in China to prevent AR, and some clinical trials have been conducted to explore its efficacy. Data from our study proved that CHM treatment was beneficial for AR treatment focusing on quality of life of AR patients, compared with placebo or some of traditional AR medicines. However, there was no significant difference between CHM and placebo or some conventional treatments in sneezing and total nasal symptoms scores.
Notably, the meta-analysis by Wang et al.10 assessing 7 RCTs suggested that CHM improved total nasal symptom scores as compared with placebo. This discordant finding between the study of Wang et al.10 and ours may be a consequence of significant heterogeneity observed in both studies and differences in the backgrounds of patients. Furthermore, subjective scoring of nasal symptoms by the patients themselves is likely to contribute to differences, and thus it should be recommended that patients in multi-centers and larger sample size are guided by 1 pre-designed rule for nasal symptom score implementation. Indeed, the meta-analysis by Wang et al.10 assessed total nasal symptom scores for symptoms, including runny nose, nasal obstruction, sneezing, itchy nose, and itchy eyes, before and after treatment, whereas no limited nasal symptoms were assessed in the present meta-analysis.
Symptom diary evaluation is commonly used in clinical trials to evaluate the efficiency of treatment strategy, with quality of life measured as a primary outcome using instruments, such as RQLQ, which includes 7 symptom measurements and SF-36 which includes 8 items.28 For a treatment strategy involving CHM, it is well known that the curative effect would be continued even after CHM treatment stopped and that prolonged administration would be required to strengthen the efficacy of CHM.2930 The present meta-analysis focused on the efficacy of CHM treatment noted at the end of the treatment period and without a follow-up period, thus limiting the data in providing information on the overall curative efficacy of the CHM treatments over longer periods. Thus, a study design investigating longer treatment periods would probably be better for revealing the full potential of CHM therapy.
Recently, treatment strategies involving combination therapy was shown to be effective in the treatment of severe AR.3132 They were based on Chinese herbs involving different mechanisms, such as immune modulation and anti-allergic ones. AR symptoms and patient drug tolerance should thus be the basis for choosing the most appropriate medical therapy.
When we meta-analyzed the CHM outcomes compared with placebo on nasal symptoms and quality of life, significant heterogeneity was observed among individual studies. One of the reasons for heterogeneity might be poor methodological quality. Moreover, it should be noted that the type of CHM employed was different among the individual studies, and treatment periods ranged from 2 to 8 weeks. In addition, the nasal symptoms and quality of life were evaluated based on 2 different evaluating systems, i.e. RQLQ and SF-36. The different severity of disease in the participants might also contribute to the risk bias of the meta-analysis.
However, this study is limited in some aspects. First, the numbers of appropriate published studies and patients' data available for inclusion and analysis in the present meta-analysis were small, and thus might influence the accuracy and interpretation of the overall findings. Secondly, there were multiple heterogeneities among the trials included, and the effect of treatment with CHB on runny nose was not assessed, which might also affect the accuracy of the results of the present study. This is important as many risk factors, for example, exposure to molds and use of antibiotics, are associated with AR prevalence and development.3334 In this regard, further studies stratified by the severity of AR, life habits, and the treatment strategy after adjusting for the background of patients are warranted. Furthermore, surgical expertise and background of enrolled patients could not be adjusted rigorously. As several CHM treatment strategies were reported in the studies included and subgroup analysis was stratified by the strategy of CHM treatment, it is possible that this might be one of the main sources of heterogeneity. Although occurrence of adverse events was one of the outcomes to be assessed in the present meta-analysis, it was not possible to perform this analysis because the occurrence of adverse events following CHM treatment was reported as an outcome measure in only one of the enrolled studies.25 This clearly suggests that further studies on CHM treatment-associated adverse events are warranted.
In summary, the present study suggests that CHM may be an effective therapy for AR. However, the small number of clinical trials available and the multiple heterogeneities noted within the trials reported suggest that the true potential of CHM as an effective therapy for AR needs to be assessed in larger, multicenter, well-controlled trials in well characterized patients treated for longer periods than currently done.
ACKNOWLEDGMENTS
This work was supported by grants from National Key R & D Program of China (2016YFC20160905200), the Program for Changjiang Scholars and Innovative Research Team (IRT13082), the National Natural Science Foundation of China (81420108009 and 81630023), the special fund of capital health development (2011-1017-06, 2011-1017-02), the special fund of sanitation elite reconstruction of Beijing (2009-2-007), Beijing health bureau program for high level talents (2011-3-043 and 2014-3-018), Beijing Municipal Administration of Hospitals' Mission Plan (SML20150203), Beijing Natural Science Foundation (7164247 and 7172053), the Capital Research Funds for Medical Development (2016-4-1092) and the priming scientific research foundation for the junior researcher in Beijing TongRen Hospital (2015-YJJ-ZZL-010).
References
1. Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016; 36:261–278. PMID: 27083101.
2. Zhang Y, Zhang L. Prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2014; 6:105–113. PMID: 24587945.

3. Zheng M, Wang X, Bo M, Wang K, Zhao Y, He F, et al. Prevalence of allergic rhinitis among adults in urban and rural areas of china: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015; 7:148–157. PMID: 25729622.

4. Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007; 62(Suppl 85):17–25.

5. Thompson A, Sardana N, Craig TJ. Sleep impairment and daytime sleepiness in patients with allergic rhinitis: the role of congestion and inflammation. Ann Allergy Asthma Immunol. 2013; 111:446–451. PMID: 24267356.

6. Kirstein MM, Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016; 32:395–400. PMID: 28229073.

7. Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012; 26:187–190. PMID: 22643942.

8. Chan RY, Chien WT. The effects of two Chinese herbal medicinal formulae vs. placebo controls for treatment of allergic rhinitis: a randomised controlled trial. Trials. 2014; 15:261. PMID: 24986270.

9. Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011; 3:103–110. PMID: 21461249.

10. Wang S, Tang Q, Qian W, Fan Y. Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy. 2012; 67:583–592. PMID: 22435619.

11. Peacock WF, Chandra A, Char D, Collins S, Der Sahakian G, Ding L, et al. Clevidipine in acute heart failure: results of the a study of blood pressure control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014; 167:529–536. PMID: 24655702.

12. Min C, Peng C, Wei G, Huang X, Fu T, Du Y, et al. Moxibustion with Chinese herbal has good effect on allergic rhinitis. Int J Clin Exp Med. 2015; 8:16480–16487. PMID: 26629174.
13. Pfaar O, Raap U, Holz M, Hörmann K, Klimek L. Pathophysiology of itching and sneezing in allergic rhinitis. Swiss Med Wkly. 2009; 139:35–40. PMID: 19169901.
14. Gu H, Jiang Z, Jiang H, Jiang Y, Zhao F, Zhang Z, et al. Review on the investigation of protective function on nervous system of compound Chinese herbal and active ingredient. J Liaoning Univ Tradit Chin Med. 2011; 10:36–38.
15. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: vol. 5. Hoboken (NJ): Wiley Online Library;2008.
16. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006; 11:193–206. PMID: 16784338.
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

18. O’Rourke K, Altman DG. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2005; 24:2733–2742. PMID: 16118810.
19. Xue CC, Thien FC, Zhang JJ, Da Costa C, Li CG. Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial. Altern Ther Health Med. 2003; 9:80–87.
20. Xue CC, Thien FC, Zhang JJ, Yang W, Da Costa C, Li CG. Effect of adding a Chinese herbal preparation to acupuncture for seasonal allergic rhinitis: randomised double-blind controlled trial. Hong Kong Med J. 2003; 9:427–434. PMID: 14660810.
21. Zhao Y, Woo KS, Ma KH, van Hansselt CA, Wong KC, Cheng KF, et al. Treatment of perennial allergic rhinitis using Shi-Bi-Lin, a Chinese herbal formula. J Ethnopharmacol. 2009; 122:100–105. PMID: 19118617.

22. Chui SH, Shek SL, Fong MY, Szeto YT, Chan K. A panel study to evaluate quality of life assessments in patients suffering from allergic rhinitis after treatment with a Chinese herbal nasal drop. Phytother Res. 2010; 24:609–613. PMID: 20014162.

23. Hsu WH, Ho TJ, Huang CY, Ho HC, Liu YL, Liu HJ, et al. Chinese medicine acupoint herbal patching for allergic rhinitis: a randomized controlled clinical trial. Am J Chin Med. 2010; 38:661–673. PMID: 20626052.

24. Hu G, Walls RS, Bass D, Ramon B, Grayson D, Jones M, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12-week clinical trial. Ann Allergy Asthma Immunol. 2002; 88:478–487. PMID: 12027069.

25. Lenon GB, Li CG, Da Costa C, Thien FC, Shen Y, Xue CC. Lack of efficacy of a herbal preparation (RCM-102) for seasonal allergic rhinitis: a double blind, randomised, placebo-controlled trial. Asia Pac Allergy. 2012; 2:187–194. PMID: 22872821.

26. Yang SH, Yu CL, Chen YL, Chiao SL, Chen ML. Traditional Chinese medicine, Xin-yi-san, reduces nasal symptoms of patients with perennial allergic rhinitis by its diverse immunomodulatory effects. Int Immunopharmacol. 2010; 10:951–958. PMID: 20546945.

27. Brinkhaus B, Hummelsberger J, Kohnen R, Seufert J, Hempen CH, Leonhardy H, et al. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004; 59:953–960. PMID: 15291903.

28. Juniper EF. Measuring health-related quality of life in rhinitis. J Allergy Clin Immunol. 1997; 99:S742–S749. PMID: 9042066.

29. Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther. 2000; 86:191–198. PMID: 10799714.

30. Xie Z. Clinical trials in traditional Chinese medicine. J Clin Ethics. 2004; 15:51–54. PMID: 15202358.
31. Krouse JH. Allergic rhinitis--current pharmacotherapy. Otolaryngol Clin North Am. 2008; 41:347–358. viiPMID: 18328373.

32. Ridolo E, Montagni M, Melli V, Braido F, Incorvaia C, Canonica GW. Pharmacotherapy of allergic rhinitis: current options and future perspectives. Expert Opin Pharmacother. 2014; 15:73–83. PMID: 24219793.

33. Kim HY. Resveratrol in asthma: a French paradox? Allergy Asthma Immunol Res. 2017; 9:1–2. PMID: 27826956.

34. Yoon J, Choi YJ, Lee E, Cho HJ, Yang SI, Kim YH, et al. Allergic rhinitis in preschool children and the clinical utility of FeNO. Allergy Asthma Immunol Res. 2017; 9:314–321. PMID: 28497918.

Fig. 2
Quality assessment of the trials included in this meta-analysis. (A) Risk of bias graph. (B) Risk of bias summary.
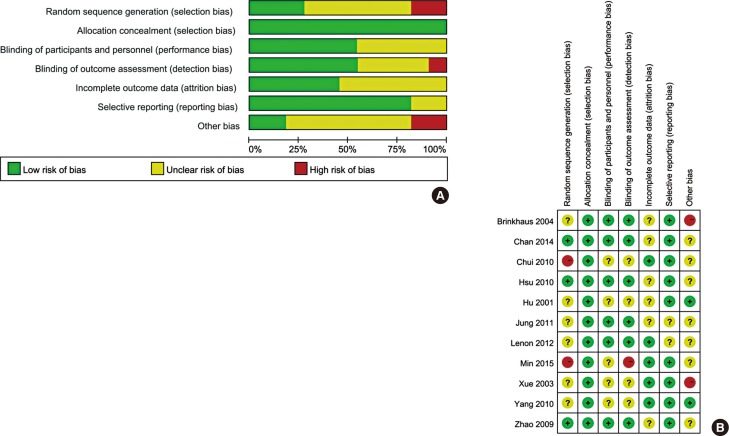
Fig. 3
Forest plot of sneezing and itchy nose based on Rhino Conjunctivitis Quality of Life Questionnaire of eligible studies comparing Chinese herbal medicine with control. (A) sneezing. (B) itchy nose.
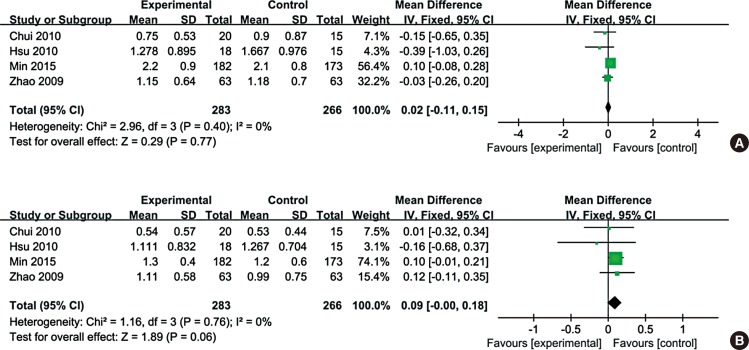
Fig. 4
Forest plot of total nasal symptom of eligible studies comparing Chinese herbal medicine with control. (A) Total nasal symptom of eligible studies comparing Chinese herbal medicine with control. (B) Total nasal symptom of eligible studies comparing Chinese herbal medicine with placebo.
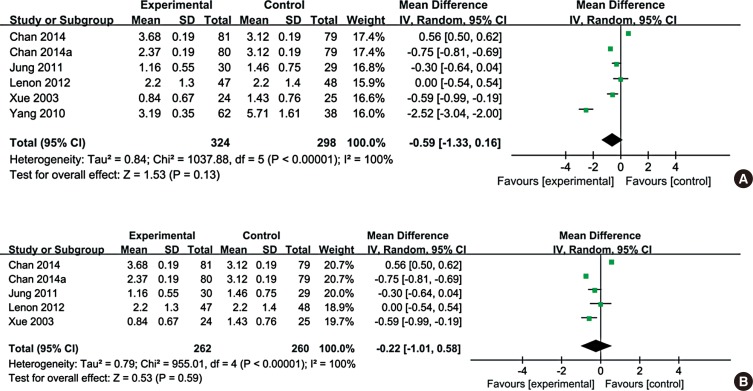
Fig. 5
Forest plot of quality of life comparing Chinese herbal medicine with control. (A) quality of life evaluation. (B) Rhino conjunctivitis Quality of Life evaluation. (C) 36-item Short Form Health Survey.
Table
Characteristics of the studies included in this meta-analysis.
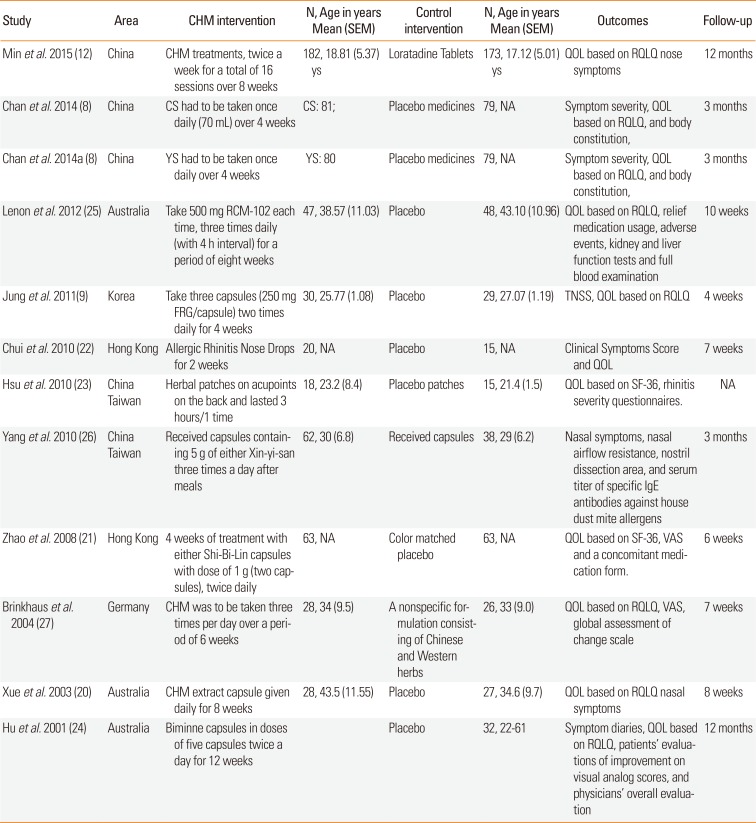
| Study | Area | CHM intervention | N, Age in years Mean (SEM) | Control intervention | N, Age in years Mean (SEM) | Outcomes | Follow-up |
|---|---|---|---|---|---|---|---|
| Min et al. 2015 (12) | China | CHM treatments, twice a week for a total of 16 sessions over 8 weeks | 182, 18.81 (5.37) ys | Loratadine Tablets | 173, 17.12 (5.01) ys | QOL based on RQLQ nose symptoms | 12 months |
| Chan et al. 2014 (8) | China | CS had to be taken once daily (70 mL) over 4 weeks | CS: 81; | Placebo medicines | 79, NA | Symptom severity, QOL based on RQLQ, and body constitution, | 3 months |
| Chan et al. 2014a (8) | China | YS had to be taken once daily over 4 weeks | YS: 80 | Placebo medicines | 79, NA | Symptom severity, QOL based on RQLQ, and body constitution, | 3 months |
| Lenon et al. 2012 (25) | Australia | Take 500 mg RCM-102 each time, three times daily (with 4 h interval) for a period of eight weeks | 47, 38.57 (11.03) | Placebo | 48, 43.10 (10.96) | QOL based on RQLQ, relief medication usage, adverse events, kidney and liver function tests and full blood examination | 10 weeks |
| Jung et al. 2011 (9) | Korea | Take three capsules (250 mg FRG/capsule) two times daily for 4 weeks | 30, 25.77 (1.08) | Placebo | 29, 27.07 (1.19) | TNSS, QOL based on RQLQ | 4 weeks |
| Chui et al. 2010 (22) | Hong Kong | Allergic Rhinitis Nose Drops for 2 weeks | 20, NA | Placebo | 15, NA | Clinical Symptoms Score and QOL | 7 weeks |
| Hsu et al. 2010 (23) |
China Taiwan |
Herbal patches on acupoints on the back and lasted 3 hours/1 time | 18, 23.2 (8.4) | Placebo patches | 15, 21.4 (1.5) | QOL based on SF-36, rhinitis severity questionnaires. | NA |
| Yang et al. 2010 (26) |
China Taiwan |
Received capsules containing 5 g of either Xin-yi-san three times a day after meals | 62, 30 (6.8) | Received capsules | 38, 29 (6.2) | Nasal symptoms, nasal airflow resistance, nostril dissection area, and serum titer of specific IgE antibodies against house dust mite allergens | 3 months |
| Zhao et al. 2008 (21) | Hong Kong | 4 weeks of treatment with either Shi-Bi-Lin capsules with dose of 1 g (two capsules), twice daily | 63, NA | Color matched placebo | 63, NA | QOL based on SF-36, VAS and a concomitant medication form. | 6 weeks |
| Brinkhaus et al. 2004 (27) | Germany | CHM was to be taken three times per day over a period of 6 weeks | 28, 34 (9.5) | A nonspecific formulation consisting of Chinese and Western herbs | 26, 33 (9.0) | QOL based on RQLQ, VAS, global assessment of change scale | 7 weeks |
| Xue et al. 2003 (20) | Australia | CHM extract capsule given daily for 8 weeks | 28, 43.5 (11.55) | Placebo | 27, 34.6 (9.7) | QOL based on RQLQ nasal symptoms | 8 weeks |
| Hu et al. 2001 (24) | Australia | Biminne capsules in doses of five capsules twice a day for 12 weeks | Placebo | 32, 22-61 | Symptom diaries, QOL based on RQLQ, patients' evaluations of improvement on visual analog scores, and physicians' overall evaluation | 12 months |
Ys, years; RQLQ, Rhino conjunctivitis Quality of Life Questionnaire; QOL, quality of life; VAS, visual analogue scale; CS, Cure-allergic-rhinitis syrup; YS, Yu-ping-feng San; TNSS, total nasal symptom score; FRG, fermented red ginseng; CHM, Chinese herbal medicine; RCM-102, RMIT Chinese Medicine 102.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download