INTRODUCTION
Allergic rhinitis (AR) is the most common respiratory allergic disease across the globe. Its prevalence, in common with other allergic diseases, such as allergic asthma (AA), has increased over the past few decades.
1 In Asia, 10% to 32% of the general population has AR
1; it is estimated to be 17% to 29% in Europe
2 and 15% in the United States have AR.
3
Allergy to house dust mites (HDM) is one of the most common causes of allergic disease. One recent study reported a rate of HDM sensitization of 89.1% in Korean children and adolescents with AR.
4 For managing HDM-induced AR, both avoidance measures and pharmacological treatment are used. Pharmacologic treatment for AR mainly includes H
1-antihistamines and leukotriene receptor antagonists (LTRA), and an intranasal corticosteroid is given to patients with more severe symptoms of AR.
5
Allergen-specific immunotherapy (AIT) is considered in patients whose symptoms are not adequately controlled with medication, in individuals experiencing side effects from medication, and those who want to avoid regular use of medication.
6 Compared with pharmacologic agents that merely offer symptomatic relief, AIT has been found to be effective in preventing sensitization to new allergens, in reducing the risk of developing asthma, and in maintaining its therapeutic effects upon treatment completion.
78 Subcutaneous immunotherapy (SCIT), 1 mode of AIT, which has been used for more than 100 years, is effective against seasonal AR, as well as perennial AR, and its efficacy is supported by several systematic reviews.
9 Compared with sublingual immunotherapy (SLIT), another mode of AIT, SCIT has been found to be more efficacious and to introduce fewer safety concerns.
9
Notwithstanding, there are several unmet needs in SCIT, especially for clinical predictors and laboratory biomarkers of efficacy.
10 In addition, the efficacy of SCIT would be unlikely to be identical in geographically different regions. Therefore, in a retrospective cohort covering 12 years, we sought to investigate clinical outcomes and prognostic factors of SCIT in Korean adults with AR.
Go to :

MATERIALS AND METHODS
Study design and population
This retrospective cohort study analyzed 304 patients who visited a university hospital from 2000 to 2012 and received SCIT with HDM with or without pollen allergens for AR for more than 1 year, but less than 7 years. The diagnosis of AR was made according to clinical symptoms, physical examination, and a skin prick test (SPT).
All patients were sensitized to HDM (Dermatophagoides pteronyssinus [Dp] and Dermatophagoides farinae [Df]) allergens (Allergopharma Joachim Ganzer KG, Reinbek, Germany) on a SPT and/or had serum specific immunoglobulin E (IgE) levels higher than 0.35 kU/L. With respect to pollens, tree (Alder, Birch, Hazel, Beech, and Oak), grass (Orchard, Rye, Bermuda, Timothy, Kentucky, and Meadow), and weed (Ragweed and Mugwort) were considered combined causative allergens of AR according to seasonal variations in rhinitis symptoms and SPT results. Positivity to allergens on SPTs was determined when the size of wheals caused by an allergen was greater than or equal to the size of wheals induced by histamine. We measured serum total and specific IgE levels to the allergens using the ImmunoCAP system (Thermo-Fisher, Uppsala, Sweden). A cutoff value of 0.35 kU/L for specific IgE was regarded as a positive result (class 1, 0.35-0.7 kU/L; class 2, 0.7-3.5 kU/L; class 3, 3.5-17.5 kU/L; class 4, 17.5-50 kU/L; class 5, 50-100 kU/L; and class 6, >100 kU/L).
Patients who were sensitized only to animal dander, mold, or pollen and who were not sensitized to HDM in SPT were excluded. Patients with asthma were also excluded in the present study. Symptom severity was classified according to medication use: patients prescribed only 1 antihistamine were considered as having mild AR; those prescribed only 1 intranasal steroid without antihistamines and LTRA, more than 2 antihistamines, or 1 antihistamine in combination with LTRA were considered as having moderate AR; and those taking 1 intranasal steroid in combination with antihistamine, and LTRA were considered as having severe AR.
11
All patients' medical records were reviewed for information on drug prescriptions, adverse events (AE) to SCIT, serum total IgE, and specific IgE to allergens included in SCIT over the treatment period. This study was approved by the Ethical Review Board of Ajou University (AJIRB-MED-MDB-15-449).
Immunotherapy
For SCIT, all patients were prescribed a Novo-Helisen Depot® (Allergopharma Joachim Ganzer KG), in which allergen extract is adsorbed to aluminum hydroxide. The SCIT treatment period comprised an initial build-up phase, followed by a maintenance phase. Immunotherapy treatment was administered via 1 of the 2 methods for increasing allergen extract in the build-up phase: conventional and rush modes. For conventional SCIT, patients received subcutaneous injections of gradually increasing doses of allergen extract every week for 12 weeks, followed by once-a-month maintenance doses. For rush SCIT, allergen extract was administered at increasing doses every 2 hours for 3 consecutive days, followed by maintenance doses every month. We divided all patients into 2 different treatment groups according to the allergen extract profile used in SCIT: HDM only and HDM+pollen.
Study outcomes
Clinical responses to SCIT were classified into 3 categories: 1) Remission was defined as no further requirement of maintenance medication (
e.g., intranasal corticosteroids or non-sedating antihistamines) for at least 1 year, as well as no record of bothersome symptoms for rhinitis on medical charts.
12 2) A controlled state was defined as patients whose symptoms were controlled well with maintenance treatment and required no further rescue medication, such as oral corticosteroids. 3) An uncontrolled state was defined as patients with poorly controlled symptoms with maintenance treatment alone, requiring oral corticosteroids and/or antibiotics more than once a year to control allergic symptoms.
AE associated with SCIT were identified upon spontaneous reports from patients, as well as through objective investigation by physicians. Systemic adverse reactions were graded according to the grading system proposed by the World Allergy Organization.
10 A single simultaneous occurrence of local and systemic AE was considered as having local and systemic AE individually. If multiple systemic AE of different grades occurred simultaneously or separately in a patient (
e.g., generalized pruritus [grade I] and laryngeal angioedema [grade III]), the one of more severe grade was recorded.
Statistical analysis
Simple cross-tabulations and descriptive statistics for the clinical characteristics of the study subjects were examined using χ2 and t tests. Rates of remission and controlled/uncontrolled states over time were determined by means of life tables and extension of survival analysis. The effects of individual parameters, such as mode of immunotherapy, target allergens, initial disease severity, and the occurrence of AE, time to remission were analyzed by Kaplan-Meier estimate and multiple logistic regression models, accommodating for both continuous and binary variables. Odds ratios (OR) are presented with 95% confidence intervals (CI). A generalized estimating equation was used to analyze temporal correlations between total IgE and specific IgE levels to HDM according to SCIT outcomes. Since serum levels of total and specific IgE did not follow normal distribution, they were converted to logarithmic values for statistical analyses. All statistical analyses were performed with SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA). P values less than 0.05 were considered statistically significant.
Go to :

RESULTS
Demographics of the study subjects
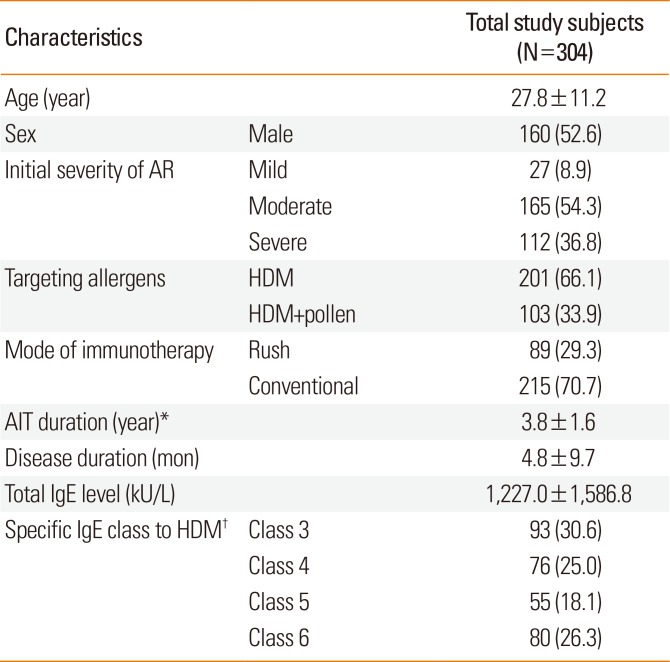
The mean age of the patients was 27.8±11.2 years, with 52.6% being male. The mean time interval between the diagnosis of AR and the commencement of SCIT at our university hospital among the study subjects was 4.8±9.7 months. Of the total subjects, 91.1% were classified as having moderate (54.3%) to severe (36.8%) AR. The mean duration of immunotherapy was 3.8±1.6 years (
Table 1).
Table 1
Demographic and clinical characteristics of the study subjects
|
Characteristics |
Total study subjects (N=304) |
|
Age (year) |
|
27.8±11.2 |
|
Sex |
Male |
160 (52.6) |
|
Initial severity of AR |
Mild |
27 (8.9) |
|
Moderate |
165 (54.3) |
|
Severe |
112 (36.8) |
|
Targeting allergens |
HDM |
201 (66.1) |
|
HDM+pollen |
103 (33.9) |
|
Mode of immunotherapy |
Rush |
89 (29.3) |
|
Conventional |
215 (70.7) |
|
AIT duration (year)*
|
|
3.8±1.6 |
|
Disease duration (mon) |
|
4.8±9.7 |
|
Total IgE level (kU/L) |
|
1,227.0±1,586.8 |
|
Specific IgE class to HDM†
|
Class 3 |
93 (30.6) |
|
Class 4 |
76 (25.0) |
|
Class 5 |
55 (18.1) |
|
Class 6 |
80 (26.3) |

Overall, 201 (66.1%) received SCIT for HDM and 103 (33.9%) for mixed allergens (at least 1 pollen with HDM). Rush SCIT was administered to 89 (29.3%) patients, while the other 215 patients received conventional SCIT. Target allergens, modes of immunotherapy, and clinical outcomes at final visit are described in
Fig. 1 in detail.
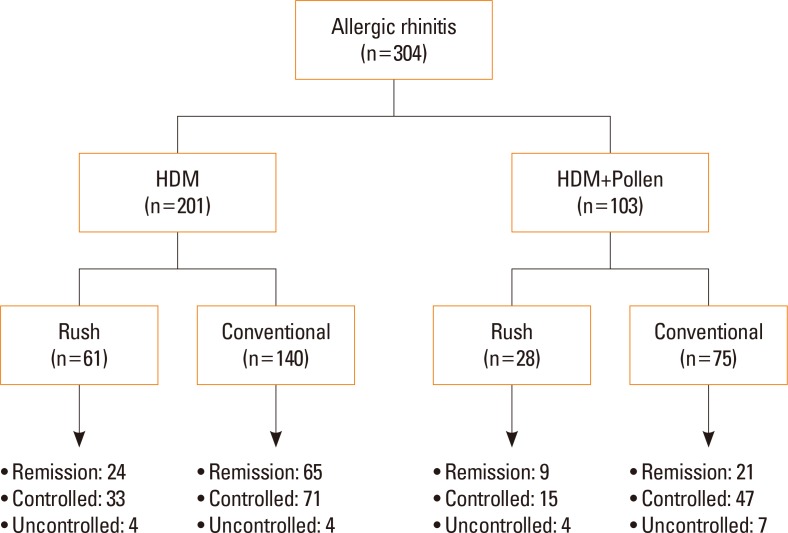 | Fig. 1Target allergens and treatment status in patients with AR. AR, allergic rhinitis; HDM, house dust mites.
|
Outcomes of immunotherapy
The cumulative incidence of clinical remission from AR was 76.6% (
Fig. 2). However, only 2.0% of patients achieved remission during the first year of SCIT. The cumulative incidence of AR remission increased annually up to 61.0% in the fifth year. The mean time until achieving remission from AR was 4.9±0.1 years, with a median of 5 years.
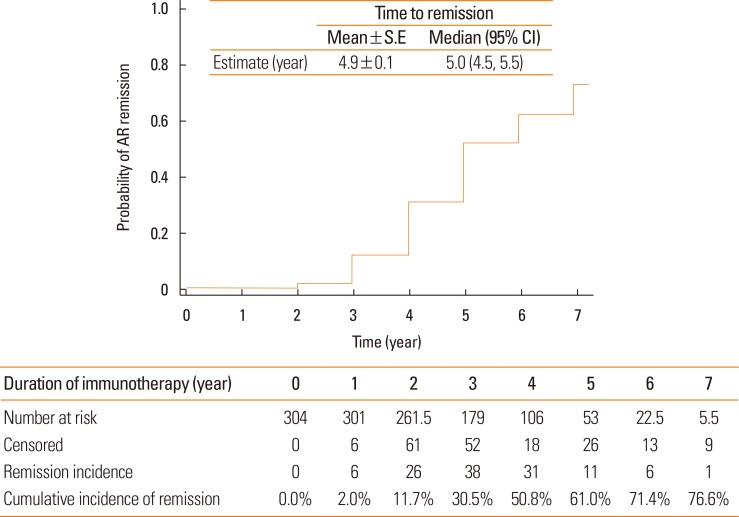 | Fig. 2Remission probability by AIT for patients with AR who were sensitized to HDM only or HDM and pollens. Survival curve and life tables were generated by the Kaplan-Meier method. AIT, allergen-specific immunotherapy; AR, allergic rhinitis; HDM, house dust mites; S.E, standard error; CI, confidence interval.
|
AE were recorded in 73 patients (24.0%). Most AE (98.6%) occurred during the build-up phase. Of the 215 patients who underwent conventional immunotherapy, 40 (18.6%) had AE, a significantly smaller proportion than the 32 of 89 (36.0%) individuals who underwent rush immunotherapy (P=0.001). Local AE occurred in 49 (16.1%), and systemic AE occurred in 72 (23.7%). The severities of the systemic AE reached grade I in 63 (20.7%), grade II in 8 (2.6%), and grade IV in 1 patient (0.3%). No patient died from an immunotherapy-related AE.
Baseline levels of total IgE and specific IgE to HDM were compared in 304 patients who received SCIT with HDM. Overall, total IgE and specific IgE levels to Dp and Df were decreased with immunotherapy. A generalized estimating equation model revealed a significant temporal correlation for total IgE levels over the SCIT period between the remission and non-remission groups of AR patients (
P=0.038); significance was not recorded for specific IgE levels to Dp or Df (
Fig. 3).
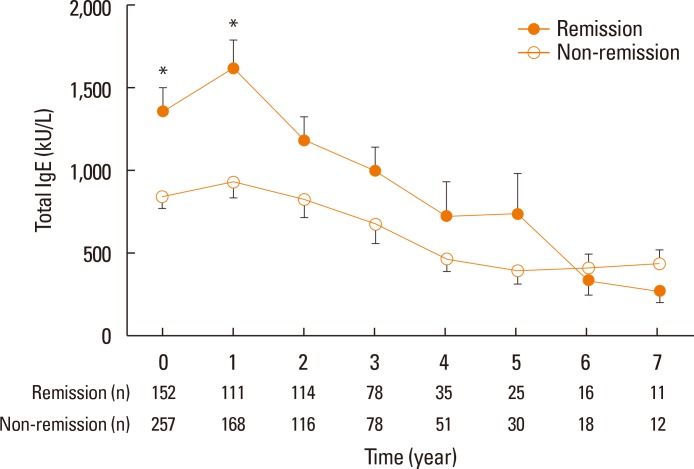 | Fig. 3A significant temporal correlation for total IgE levels over the SCIT period between the remission and non-remission groups of AR patients was indicated in a generalized estimating equation model. Error bars indicate standard error means of total serum IgE levels. IgE, immunoglobulin E; SCIT, subcutaneous immunotherapy; AR, allergic rhinitis. *P<0.05.
|
Changes in skin reactivity were evaluated according to differences in the A/H ratio (the ratio of mean wheal diameters induced by HDM allergens and histamine [1 mg/mL] on the SPT) at baseline and upon completion of AIT in 121 patients with HDM-sensitized AR. A/H ratios at the completion of AIT were significantly decreased for both Dp (3.9±2.2 vs 2.3±1.6, P< 0.001) and Df (3.3±2.2 vs 1.9±1.1, P=0.002). Mean reductions in the A/H ratio for HDM allergens were not different between the remission and non-remission groups (38.2%±43.65% vs 23.9%±60.9%, P=0.185).
Predictive factors for clinical responses to immunotherapy
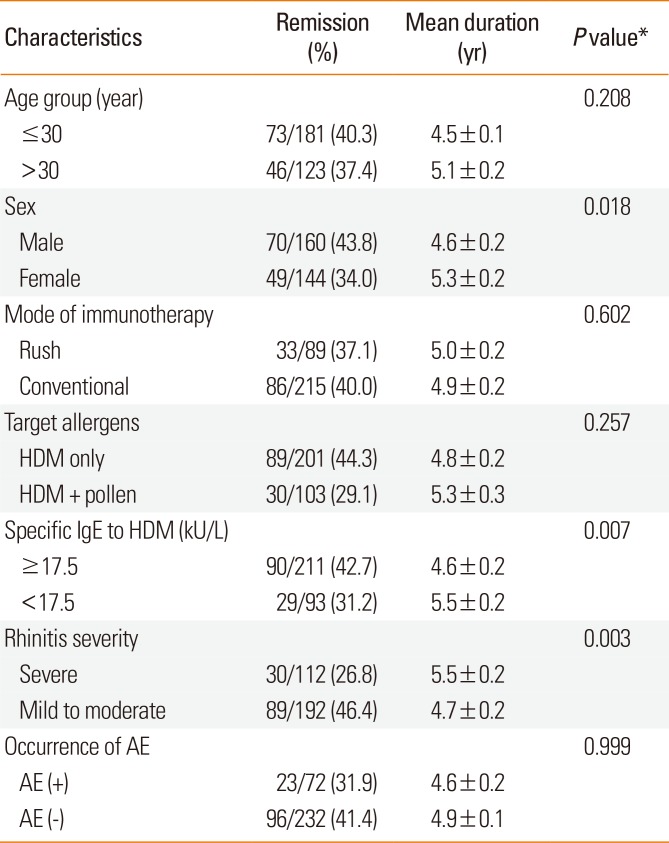
Age, mode of immunotherapy, target allergens, and occurrence of AE did not affect remission rates or the duration of immunotherapy until remission from AR (
Table 2). Male patients had more favorable results than females in terms of remission rate and duration of immunotherapy until remission (43.8% vs 34.0%, 4.6±0.2 vs 5.3±0.2 years,
P=0.018). When patients were divided into 2 groups according to specific IgE levels to HDM, those with IgE levels ≥17.5 kU/L were found to have benefited more from SCIT in terms of remission than those with IgE levels <17.5 kU/L (42.7% vs 31.2%, 4.6±0.2 vs 5.4±0.2 years,
P=0.007). A higher remission rate for AR was observed in patients with higher classes of HDM-specific IgE at the start of SCIT: 31.2% (29/93) in class 3, 35.5% (27/76) in class 4, 40.0% (22/55) in class 5, and 51.2% (41/80) in class 6, which showed significant differences (
P=0.006, linear-by-linear association). Patients with mild to moderate AR showed an increased remission rate and a shorter period of immunotherapy than those with severe AR (46.4% vs 26.8%, 4.7±0.2 vs 5.4±0.2 years,
P=0.003).
Table 2
Remission rate and mean duration until remission in patients with AR treated with allergen specific SCIT
|
Characteristics |
Remission (%) |
Mean duration (yr) |
P value*
|
|
Age group (year) |
|
|
0.208 |
|
≤30 |
73/181 (40.3) |
4.5±0.1 |
|
>30 |
46/123 (37.4) |
5.1±0.2 |
|
Sex |
|
|
0.018 |
|
Male |
70/160 (43.8) |
4.6±0.2 |
|
Female |
49/144 (34.0) |
5.3±0.2 |
|
Mode of immunotherapy |
|
|
0.602 |
|
Rush |
33/89 (37.1) |
5.0±0.2 |
|
Conventional |
86/215 (40.0) |
4.9±0.2 |
|
Target allergens |
|
|
0.257 |
|
HDM only |
89/201 (44.3) |
4.8±0.2 |
|
HDM + pollen |
30/103 (29.1) |
5.3±0.3 |
|
Specific IgE to HDM (kU/L) |
|
|
0.007 |
|
≥17.5 |
90/211 (42.7) |
4.6±0.2 |
|
<17.5 |
29/93 (31.2) |
5.5±0.2 |
|
Rhinitis severity |
|
|
0.003 |
|
Severe |
30/112 (26.8) |
5.5±0.2 |
|
Mild to moderate |
89/192 (46.4) |
4.7±0.2 |
|
Occurrence of AE |
|
|
0.999 |
|
AE (+) |
23/72 (31.9) |
4.6±0.2 |
|
AE (−) |
96/232 (41.4) |
4.9±0.1 |

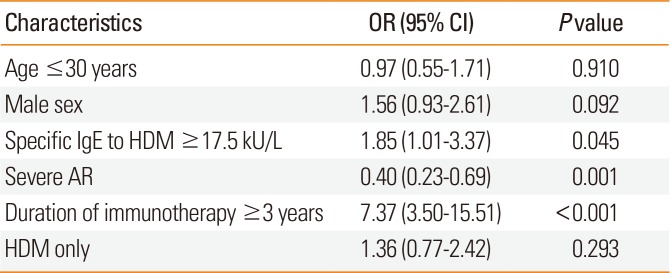
In multiple logistic regression analysis, specific IgE to HDM ≥ class 4 (OR, 1.85; 95% CI, 1.01-3.38;
P=0.045), duration of SCIT ≥3 years (OR, 7.37; 95% CI, 3.50-15.51;
P<0.001), and severe AR (OR, 0.40; 95% CI, 0.23-0.69;
P=0.001) were identified as significant and independent predictors of clinical remission in patients with AR undergoing AIT after adjustment for age and sex (
Table 3).
Table 3
ORs of characteristics related with clinical remission after immunotherapy by means of logistic regression analysis in AR patients
|
Characteristics |
OR (95% CI) |
P value |
|
Age ≤30 years |
0.97 (0.55–1.71) |
0.910 |
|
Male sex |
1.56 (0.93–2.61) |
0.092 |
|
Specific IgE to HDM ≥17.5 kU/L |
1.85 (1.01–3.37) |
0.045 |
|
Severe AR |
0.40 (0.23–0.69) |
0.001 |
|
Duration of immunotherapy ≥3 years |
7.37 (3.50–15.51) |
<0.001 |
|
HDM only |
1.36 (0.77–2.42) |
0.293 |

Comparative analysis between SCIT with a single allergen and with multiple allergens in multi-sensitized patients
Multi-sensitization was defined as sensitivity to both HDM and at least 1 pollen allergen. Of 111 AR patients who were sensitized to both HDM and any pollen, 66 underwent SCIT with HDM alone, while the other 45 patients underwent SCIT with multiple allergens. A higher remission rate and a shorter maintenance period of SCIT until remission were noted in multi-sensitized patients who underwent SCIT with HDM alone, compared to those who underwent immunotherapy with multiple allergens, although the differences were not statistically significant (45.5% vs 31.1%, 4.4±0.2 vs 5.3±0.4 years, P=0.311).
Go to :

DISCUSSION
In the present retrospective study, SCIT with aluminum hydroxide-adsorbed allergen extract facilitated remission in 76.6% of patients with AR within 4.9 years on average. IgE levels specific to HDM were identified as significant predictors of favorable responses to SCIT. Moreover, in multi-sensitized patients, clinical responses did not differ significantly between patients undergoing SCIT with multiple allergens and those undergoing SCIT with HDM alone.
Previous guidelines have recommended that an age of at least 5 years is safe for immunotherapy, with no upper limit for age.
6 In our study, age at starting SCIT had no significant influence on clinical outcomes, although a tendency toward more favorable responses was noted in younger patients at ages ≤30 years. Corresponding with our results, previous studies have reported no significant difference between treatment response and age.
1314 In another study, the clinical efficacy of treatment in patients older than 54 years was not different from that in patients younger than 54 years.
15 On the contrary, a recent study has indicated that patients with a shorter symptom duration of AR experience greater efficacy from AIT.
16 Since data with which to conclude this relationship are insufficient, it remains to be investigated.
In the present study, we noted that the effect of AIT decreased in severe AR patients. Schmitt et al.
11 previously described the preventive effects of immunotherapy from AR to AA in a large retrospective cohort study of antihistamine prescriptions and health care use as a surrogate marker of severity, similar to how we classified patients in our study. Although the primary endpoint was different from our study, patients with more severe AR tended to progress to AA more frequently despite receiving immunotherapy.
11 Meanwhile, however, other studies have suggested that highly symptomatic patients appear to benefit more from AIT than their counterparts.
1617 These conflicting reports in regards to the effects of AIT in relation to severity might be based on adoption of different ways of classifying severity (
i.e., by either medication use or symptoms).
Predicting individuals who will respond favorably to immunotherapy has been a major concern and unmet need.
18 Specific IgE levels seem to be a promising biologic marker to fulfill this demand. Ciprandi and Silvestri
19 suggested a cutoff value of >9.74 kU/L for
Parietaria judaica, HDM, and birch to discriminate between responders and non-responders among patients with rhinitis and/or asthma. In addition, Tosca et al.
20 described that allergic children with specific IgE to HDM >10 kU/L showed more favorable results than those with levels <10 kU/L. In the present study, specific IgE to HDM ≥ class 4 or specific IgE levels to HDM ≥17.5 kU/L at the start of SCIT were significantly associated with clinical remission of AR in adult patients who were sensitized to HDM and/or pollens. Generally, levels of specific IgE in serum reflect the degree of exposure to particular allergens. The dose-dependent association between HDM-specific IgE levels and allergen-related symptoms has been reported in a prior study.
2122 Therein, the odds of dust-related symptoms increased by 5-fold from subjects not sensitized to HDM to subjects with HDM-specific IgE levels ≥17.5 kU/L.
21 They also found that subjects with higher levels of specific IgE more frequently reported using inhalers.
21 Thus, it is believable that the higher the specific IgE level, the more clinically relevant the allergen is for a particular individual. The more clinically associated the allergens for AIT, the more effective outcomes will be acquired. In the same context, Di Lorenzo et al.
23 demonstrated that both serum specific IgE level and specific IgE to total IgE ratio are significantly correlated with clinical responses to AIT. A possible mechanism is that the induction of regulatory T cells specific to the dominant allergen of a particular patient can help suppress IgE responses to relevant allergens during AIT, resulting in an overall decrease in allergenic inflammation.
23 Thus, baseline levels of specific IgE to HDM may be an acceptable predictor of effective immunotherapy in AR patients, although more validation and a clear cutoff level are still needed.
A previous study estimated that approximately 80% of allergic patients are polysensitized, which makes it difficult to select allergen extracts for immunotherapy.
24 Although the US has favored multi-allergen immunotherapy and European countries have preferred immunotherapy with a single or a few relevant allergens, it is impossible to directly compare which is more effective because of regional differences in clinically relevant allergens and the lack of standardization of allergen extracts.
6 Single-allergen immunotherapy for seasonal grass pollen and perennial HDM has been found to be equally effective in monosensitized and polysensitized patients.
25 However, few studies have attempted to investigate efficacy between single-allergen and multi-allergen AIT within polysensitized patients. Although we could not draw a definitive conclusion on this concern, our results showed that single-allergen immunotherapy with HDM only was enough to achieve remission or at least better than multi-allergen AIT when treating patients polysensitized to HDM and pollens. In support of our results, a recent study described that multi-allergen immunotherapy with seasonal and perennial allergens failed to prevent AR from progressing to AA, whereas single-allergen immunotherapy did.
11 Taken together, these results suggest that multi-sensitized patients with AR can be effectively treated with immunotherapy targeting only HDM rather than multiple allergens. Indeed, when mixing allergens, unnecessary dilution or proteolysis of allergens can occur, potentially reducing the efficacy thereof in AIT.
6
The present study has several limitations. In our retrospective cohort analysis, the severity of AR was defined by medication requirements not by clinical symptoms. In general, clinical trials to prove the efficacy of AIT in AR patients have adopted total symptom scores and total medication scores as primary end-points. However, it is difficult for clinicians to evaluate these patient-oriented outcome measures prospectively in routine clinical practice. Also, the lack of a control group that did not receive SCIT made it difficult to estimate the true effectiveness of AIT. The present study, nonetheless, also has some merit in that clinical outcomes (remission and control states) were applied as primary endpoints in a relatively large population and analyzed in terms of remission rate over time. Also, the study covers maintenance durations of up to 7 years in patients from a single institution.
In conclusion, this large retrospective cohort expounded on previous results by demonstrating that AIT facilitates remission in 76.6% of adult AR patients sensitized to HDM with rare serious AE. Finally, specific IgE levels to HDM were found to influence clinical responses to SCIT in Korean patients with AR.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download