Abstract
Purpose
Dyspnea is not widely utilized as an indicator of asthma provocation despite its universal presentation. We hypothesized that dyspnea severity was proportionate with the lung function decline, methacholine dose-step, and the degree of bronchial hyperresponsiveness (BHR).
Methods
We retrospectively analyzed 73 children's bronchial provocation test data with an assessment of dyspnea at every dose-step. Dyspnea severity was scored using a modified Borg (mBorg) scale. A linear mixed effect analysis was performed to evaluate the relationship between the mBorg scale, the percentage fall in the forced expiratory volume in 1 second (FEV1) (ΔFEV1%), the methacholine dose-step, and the degree of BHR (BHR grade).
Results
Subjects were divided into 5 BHR groups based on their last methacholine dose-steps. The mBorg scores did not differ significantly among BHR groups (P=0.596, Kruskal-Wallis test). The linear mixed effect analysis showed that ΔFEV1% was affected by the methacholine dose-step (P<0.001) and BHR grade (P<0.001). The mBorg score was affected by the dose-step (P<0.001) and BHR grade (P=0.019). We developed a model to predict the mBorg score and found that it was affected by the methacholine dose-step and ΔFEV1%, elevating it by a score of 0.039 (χ2 [1]=21.06, P<0.001) and 0.327 (χ2 [1]=47.45, P<0.001), respectively. A significant interaction was observed between the methacholine dose-step and ΔFEV1% (χ2 [1]=16.20, P<0.001).
Conclusions
In asthmatic children, inhaled methacholine, as well as the degree of BHR and lung function decline, may affect dyspnea perception during the bronchial provocation test. If we wish to draw meaningful information from dyspnea perception, we have to consider various complicating factors underlying it.
Dyspnea is an unpleasant sensation of labored or difficult breathing.1 It is one of the cardinal symptoms of asthma, along with cough, wheezing, and chest tightness. Furthermore, among asthmatic patients who visited the emergency department because of increased symptom severity, dyspnea was the most commonly reported complaint.2 Recurrent episodes of dyspnea or other relevant symptoms indicating airway obstruction are essential for the diagnosis of asthma, as well as the exclusion of other possible causes.3
The bronchial provocation test is indicated to replicate asthma presentation by stimulating bronchial contraction. By gradually increasing the stimulus, we can verify the presence of bronchial hyperresponsiveness (BHR), which is a fall in the forced expiratory volume in 1 second (FEV1) beyond a certain threshold in asthmatic subjects.45 In general, the amount of stimulus that can lead to a >20% bronchial obstruction is regarded as an indicator of BHR.6 In young children, bronchial obstruction can be assessed using the relevant physical findings, typically the emergence of wheezing or desaturation. However, dyspnea is not widely utilized as an indicator of asthma provocation despite its universal presentation. Additionally, it is controversial whether the degree of dyspnea is proportionate with lung function decline, or whether the relationship varies according to the degree of BHR (BHR grade).789
In this study, we intended to explore the clinical significance of dyspnea during induced bronchoconstriction. We measured changes in the severity of dyspnea during bronchial provocation tests in children with asthma and evaluated their association with lung function reduction. We aimed to verify whether dyspnea perception correlates with lung function decline and whether there are other variables that affect dyspnea perception.
We retrospectively analyzed deidentified data from another study that evaluated the relationship between a modified Borg (mBorg) scale and the pictogram-based dyspnea scale during induced bronchoconstriction in children with clinical asthma.10 (approval No. H-1302-019-462) The study recruited 73 asthmatic children, aged ≥6 years, who visited the allergy clinic in our institute. All subjects had a history of clinical asthma, such as chronic cough, episodic dyspnea, and/or wheezing within the past year. Patients with a history of near-fatal asthma, major exacerbations necessitating the use of systemic corticosteroids, or serious respiratory diseases other than asthma, such as bronchopulmonary dysplasia or bronchiectasis, were excluded from the study. This analysis was approved by the Institutional Ethics Committee of the Seoul National University Hospital/Seoul National University College of Medicine (approval No. H-1704-112-847) and the requirement for consent was waived.
Forced vital capacity (FVC) and FEV1 were measured via forced expiratory maneuvers according to standards that met the American Thoracic Society (ATS) criteria11 using a spirometer (MicroPlus; CareFusion, Basingstoke, UK). All subjects were requested to stop taking inhaled corticosteroids and leukotriene receptor antagonists for 2 weeks, antihistamines for 1 week, bronchodilators and other medications for 48 hours, and short-acting β2-agonists for 8 hours before the tests. None of our subjects had any symptoms of upper respiratory tract infections in the 2 weeks preceding the tests. Before we proceeded to perform the bronchial challenges, the patients were trained to conduct spirometry in a reproducible way (i.e., a coefficient of variation of FEV1 in 3 consecutive flow-volume curves of <5%), and they were required to have an FEV1 of at least 70% of the predicted value.
The bronchial provocation test was carried out according to the ATS guidelines.6 Fresh solutions of methacholine chloride (Sigma-Aldrich, St. Louis, MO, USA) were prepared in buffered saline solution at concentrations of 0.625, 2.5, 5, 25, and 100 mg/mL. We could not apply Provocholine® (methacholine chloride USP; Methapharm Inc., Ontario, Canada) because it was commercially unavailable in our country during the study period. First, the subjects were instructed to inhale a dose of 0.9% saline 5 times. After a 2-minute interval, we assessed the subjects' dyspnea and FEV1, and recorded these measurements as the baseline readings. These measurements were then repeated 2 minutes after 5 inhalations of doses of 0.625, 2.5, 5, 25, and 100 mg/mL methacholine. The test was terminated if FEV1 showed a decrease of more than 20% compared to the baseline value, or if FEV1 did not decrease by more than 20% at the last dose-step. For subjects who exhibited a percentage fall in the FEV1 from baseline (ΔFEV1%) of ≥20%, the provocative concentration of methacholine causing the ΔFEV1% of 20% (PC20) was calculated by interpolation from a log-linear graph.
During the bronchial challenges, the severity of perceived dyspnea was assessed by the mBorg scale12 and the pediatric dyspnea scale (PDS).13 The Borg scale is a vertical list with labeled categories (0-10) describing increasing intensities of dyspnea (0 “nothing at all” and 10 “maximal”). We used a modified version of the Borg scale that was translated into the Korean language. Because the Korean version of the mBorg scale has previously been validated but the PDS has not, we selected the mBorg scale to assess the relationship with lung function parameters. Prior to the bronchial provocation, we explained the mBorg scale to the participants and gave them time to familiarize themselves with the scale. Before FEV1 measurement on each dose-step, we asked the children “How severe was your breathlessness after this inhalation?” We did not remind subjects of their dyspnea scores in the previous step. Throughout the provocation test, the subjects were blinded to their lung function results.
The R statistical software (version 3.3.2; R Project for Statistical Computing, Vienna, Austria), are used for data analysis. Continuous variables, such as age, height, weight, FEV1, and ΔFEV1% were presented as the mean±standard deviation. Dyspnea scores are presented as the median and the interquartile range. The Kruskal-Wallis test was utilized to assess the group difference in the medians of the last dose-step mBorg scores; the trend of these scores across the BHR groups was examined by the Jonckheere-Terpstra test.14 A linear mixed model analysis15 was performed to evaluate the relationship among the mBorg, ΔFEV1%, methacholine dose-step, and BHR grade. P values were obtained using a likelihood ratio test of the full model with the effect in question against the model without the effect in question. The significance level was statistically significant when a P value was less than 0.05.
A total of 73 asthmatic children underwent the methacholine provocation test. Table presents their baseline characteristics, including the profile of BHR grades and mBorg scores. The mean age of the participants was 10.8 years (48% [35/73] of them aged <10 years) and 56.2% were male. Forty-three subjects (43/73, 58.9%) presented significant bronchoconstriction, defined as a ΔFEV1% ≥20% on any dose-step. Thirty children had a ΔFEV1% of <20% even after undergoing the final dose-step of the bronchial challenges. If we define BHR as the PC20 <16 mg/mL, 28 subjects (28/73, 38.4%) had BHR. We then assigned a BHR grade to all the subjects, based on the dose-step that they reached during the test (Table). This classification was performed as follows: group I (n=6) with ΔFEV1% > 20% at the first dose-step, indicating severe BHR; group II (n=4) with ΔFEV1% >20% at the second dose-step; and similarly, for groups III (n=5) and IV (n=18). Group V (n=40) consisted of those subjects whose ΔFEV1% remained <20% after the fourth dose-step and ended up receiving the final dose-step.
There was no significant difference in the median last dose-step mBorg scores between subjects who had BHR and those who did not (P=0.272), neither was there a significant difference among the 5 BHR groups (P=0.596). The median of last dose-step mBorg scores increased from 2 to 4 as the group number increased from I to IV (Table), whereas the trend was not statistically significant, even when we confined the analysis to subjects in groupd I to IV, i.e., those who exhibited significant bronchoconstriction at methacholine concentrations <25 mg/mL (n=33, P=0.405, Jonckheere-Terpstra test).
Fig. 1 depicts the changes in ΔFEV1% across dose-steps with regard to the subjects' BHR group. The linear mixed effect analysis showed that the methacholine dose-step affected ΔFEV1% (χ2 [1]=233.48, P<0.001), increasing by 3.90±0.21 (standard error). The BHR grades also affected ΔFEV1% (χ2 [1]=96.47, P<0.001), decreasing by -4.87±0.40.
The changes in mBorg scores for increasing dose-steps with regard to the subjects' BHR group are shown in Fig. 2. Except for one point (BHR group IV at a methacholine concentration of 2.5 mg/mL), the median mBorg score increased with the dose-steps in all BHR groups. For a given dose-step, the median mBorg score decreased when the BHR group number increased. The linear mixed effect analysis showed that the methacholine dose-step affected the mBorg scores (χ2 [1]= 162.88, P<0.001), increasing by 0.480±0.033 (standard error). The BHR grades also affected the mBorg scores (χ2 [1]=5.43, P=0.020), decreasing by -0.252±0.106.
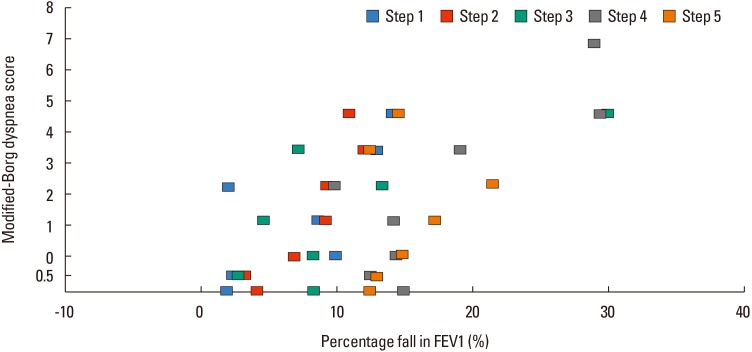
Fig. 3 presents the distribution of mBorg scores according to ΔFEV1% by methacholine dose-steps. When we developed a model to predict mBorg scores by ΔFEV1%, dose-step, and BHR grades, we found that the ΔFEV1% and methacholine dose-step affected the mBorg score, elevating it by 0.039 (ΔFEV1%, χ2 [1]=21.06, P<0.001) and 0.327 (methacholine dose-step, χ2 [1]=47.45, P<0.001), respectively. BHR grades, however, did not show a significant contribution (χ2 [1]=0.315, P=0.574). A significant interaction was observed between the methacholine dose step and ΔFEV1% (χ2 [1]=16.20, P<0.001).
When assessing the degree of dyspnea in asthmatic children during induced bronchoconstriction, we noticed a trend of decreasing lung function and increasing degrees of dyspnea as the dose-steps increased. We also observed that the trend was more evident when children had more severe BHR. It was a universal finding that subjects perceived more severe dyspnea as their lung function became worse, regardless of BHR status. One interesting finding was that the dose-step was independently associated with dyspnea perception. This study was the first to evaluate the complex relationships among dyspnea perception, lung function decrease, degree of BHR, and methacholine dose-step during the provocation test in children with clinical asthma.
Asthmatic children specifically present with dyspnea, wheezy breathing, and lung function decline when exposed to bronchial stimulants. The latter 2 items have been widely used to assess BHR;616 however, dyspnea is seldom utilized as a means to assess BHR. This may be because dyspnea perception is multi-dimensional and difficult to score by a single scale, too obscure to verify via replication, or too subjective to allow for precise comparisons between subjects.17 A previous study evaluated the degree of dyspnea in asthmatic children and reported that dyspnea assessed by the visual analog scale (VAS) in real life differs according to airway obstruction defined by FEV1 values <80% of the predicted values.18 Another study suggested a VAS score of ≥6 as a reliable cutoff for subjects who perceive asthma symptoms in adult asthmatics.19 These studies consistently show that subjects with poorer lung function exhibit a higher degree of dyspnea, which was in accordance with the findings in the present study. However, these studies did not give any information on the subjects' inherent BHR, which we assessed during the bronchial provocation test.
Regarding dyspnea measured during induced bronchoconstriction, most studies have focused only on the dyspnea perception score at a 20% decrease in FEV1 relative to baseline (PS20).202122 As an index of the awareness of asthma symptoms, PS20 is useful in differentiating between hypo-, normo-, or hyper-perceivers. Identifying children's perceiving patterns can help precisely interpret the level of control and prescribe appropriate controller medications.21 However, PS20 does not cover subjects with BHR-negative clinical asthma whose PS20 cannot be calculated and it does not contain information on each subject's degree of BHR. One study calculated the Borg slope by plotting each subject's Borg scores against their fall in FEV1.22 The Borg slope presented good associations with the baseline lung function and the cumulative dose of bronchial stimulant causing a 20% fall in FEV1. These studies consistently found that BHR correlates with symptom perception, in other words, children with more severe BHR would perceive their dyspnea as less severe.
In the present study, we focused on the multi-dimensional aspect of dyspnea. When we confined the analysis to subjects with BHR-negative clinical asthma, the median dyspnea score ranged from 0.5 to 3, although these subjects had no significant lung function decline. Moreover, they presented a trend of increasing dyspnea in accordance with increasing dose-steps. Because an increase in dose-step involves exposure to more stimulant for a longer time, the increasing dose-steps themselves may have affected the subjects psychologically, or the bronchial stimulant inhaled during the challenge tests may have caused unpleasant feelings in the asthmatic children. Therefore, we speculated that the increase in dose along with the longer duration of exposure may have confounded their dyspnea perception and even reversed the dyspnea perception related to BHR. Indeed, when we divided the subjects according to their BHR grades, we found a trend of more severe dyspnea perception corresponding to more severe BHR, which is the opposite of previous findings. This discrepancy points toward the complexity of dyspnea perception, which is the sum of various factors, such as lung function decline, amount of inhaled stimulant, and subjects' degree of BHR at the time of bronchial constriction.
Among various indices of dyspnea, we selected the mBorg scale because it was widely used in assessing PS20 in previous studies.21222324 However, the Borg scale was not invented specifically for asthmatic subjects, but rather intended for assessing fatigue by any cause. Furthermore, pediatric patients might not fully understand the difference in degrees of dyspnea when it is expressed in words rather than through the VAS.252627 Moreover, it is still unclear whether children are impaired in this complex process of cognition, interpretation, and scoring, and, if yes, how much they are impaired.
The present study is a re-analysis of data from another study that aimed to validate a dyspnea scale complemented by pictograms.10 Unfortunately, we did not collect information on clinical severity or the duration of asthma symptoms when enrolling subjects in the initial study. This would have allowed us to estimate the proportion of chronic severe asthmatics, which may have influenced the association between the bronchoconstriction and dyspnea perception. Moreover, the season of bronchial challenges or the time elapsed since the subjects' last exacerbation may have influenced their asthma control status and affected the relationship between dyspnea and the other pulmonary function parameters.2829 Children with severe persistent asthma typically have blunted dyspnea perception due to their declined lung function.82230
In this study, we investigated dyspnea intentionally induced by a nonspecific and direct stimulus. In reality, dyspnea perceived during asthma exacerbation might originate from different sources. Therefore, the generalizability of our findings to asthmatic children experiencing natural exacerbation is limited. However, our findings do suggest that PS20 values are insufficient for categorizing asthmatic subjects; clinicians should take into account the degree of BHR. Further studies on this topic should adopt an exercise challenge test rather than a methacholine test, because such graded challenges cannot be free from the psychological issues that accompany repeated procedures. More clinical information, including the asthma duration, clinical severity, and other demographic variables, would also be helpful to adjust for confounding factors.
In conclusion, the evaluation of dyspnea perceived during induced bronchoconstriction is complex. In asthmatic children, inhaled methacholine, as well as the degree of BHR and lung function decline, may affect dyspnea perception during the bronchial provocation test. Contrary to previous findings, more severe BHR presents as more severe dyspnea when the effect of the methacholine inhaled during the increased dose-steps is considered. If we wish to draw meaningful information from an assessment of dyspnea perception, we have to consider various complicating factors underlying it.
ACKNOWLEDGMENTS
Dong In Suh takes responsibility for the content of the manuscript, including the data and the analysis. Dong In Suh developed the aims for the presented analyses. Yun Jung Choi carried out the initial and final analyses with assistance from Dong In Suh. Yun Jung Choi and Dong In Suh drafted the initial and final manuscript. Young Yull Koh and Myung Hyun Sohn conceived and designed the study. All authors participated in manuscript preparation and approved the final version of the manuscript.
References
1. Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000; 11:2117–2120. PMID: 10923655.

2. Fitzgerald JM, Hargreave FE. Acute asthma: emergency department management and prospective evaluation of outcome. CMAJ. 1990; 142:591–595. PMID: 1968778.
3. Parente AA, March MF, Evangelista LA, Cunha AL. Perception of dyspnea in childhood asthma crisis by the patients and those in charge of them. J Pediatr (Rio J). 2011; 87:541–546. PMID: 22170258.

4. Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis. 1980; 121:389–413. PMID: 6987924.
5. Rosenthal RR, Norman PS, Summer WR, Permutt S. Role of the parasympathetic system in antigen-induced bronchospasm. J Appl Physiol Respir Environ Exerc Physiol. 1977; 42:600–606. PMID: 863822.

6. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329. PMID: 10619836.
7. Baker RR, Mishoe SC, Zaitoun FH, Arant CB, Lucas J, Rupp NT. Poor perception of airway obstruction in children with asthma. J Asthma. 2000; 37:613–624. PMID: 11059529.

8. Motomura C, Odajima H, Tezuka J, Harada J, Okada K, Nishima S. Perception of dyspnea during acetylcholine-induced bronchoconstriction in asthmatic children. Ann Allergy Asthma Immunol. 2009; 102:121–124. PMID: 19230462.

9. Panditi S, Silverman M. Perception of exercise induced asthma by children and their parents. Arch Dis Child. 2003; 88:807–811. PMID: 12937106.

10. Kim YS, Shin JM, Choi YJ, Suh DI, Koh YY. Comparison on the profiles of the two dyspnea score during an induced bronchoconstriction in children with clinical asthma. Allergy Asthma Respir Dis. 2017; 5:262–269.
11. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338. PMID: 16055882.
12. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14:377–381. PMID: 7154893.

13. Khan FI, Reddy RC, Baptist AP. Pediatric Dyspnea Scale for use in hospitalized patients with asthma. J Allergy Clin Immunol. 2009; 123:660–664. PMID: 19181371.

14. Lunneborg CE. Jonckheere–Terpstra test. In : . Hoboken (NJ): John Wiley & Sons;2014.
15. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015; 67:1–48.
16. Avital A, Bar-Yishay E, Springer C, Godfrey S. Bronchial provocation tests in young children using tracheal auscultation. J Pediatr. 1988; 112:591–594. PMID: 3280772.

17. Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009; 167:53–60. PMID: 18706531.

18. Tosca MA, Silvestri M, Olcese R, Pistorio A, Rossi GA, Ciprandi G. Breathlessness perception assessed by visual analogue scale and lung function in children with asthma: a real-life study. Pediatr Allergy Immunol. 2012; 23:537–542. PMID: 22625869.

19. Ciprandi G, Schiavetti I, Sorbello V, Ricciardolo FL. Perception of asthma symptoms as assessed on the visual analog scale in subjects with asthma: a real-life study. Respir Care. 2016; 61:23–29. PMID: 26420899.

20. Burdon JG, Juniper EF, Killian KJ, Hargreave FE, Campbell EJ. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982; 126:825–828. PMID: 7149447.
21. Chetta A, Castagnaro A, Foresi A, Del Donno M, Pisi G, Malorgio R, et al. Assessment of breathlessness perception by Borg scale in asthmatic patients: reproducibility and applicability to different stimuli. J Asthma. 2003; 40:323–329. PMID: 12807177.

22. Cato Study Group. Nuijsink M, Hop WC, Jongste JC, Sterk PJ, Duiverman AE. Perception of bronchoconstriction: a complementary disease marker in children with asthma. J Asthma. 2013; 50:560–564. PMID: 23672570.

23. Boulet LP, Leblanc P, Turcotte H. Perception scoring of induced bronchoconstriction as an index of awareness of asthma symptoms. Chest. 1994; 105:1430–1433. PMID: 8181331.

24. Turcotte H, Corbeil F, Boulet LP. Perception of breathlessness during bronchoconstriction induced by antigen, exercise, and histamine challenges. Thorax. 1990; 45:914–918. PMID: 2281422.

25. van Gent R, van Essen-Zandvliet LE, Rovers MM, Kimpen JL, de Meer G, van der Ent CK. Poor perception of dyspnoea in children with undiagnosed asthma. Eur Respir J. 2007; 30:887–891. PMID: 17652315.

26. Yoos HL, McMullen A. Symptom perception and evaluation in childhood asthma. Nurs Res. 1999; 48:2–8. PMID: 10029396.

27. Fritz GK, McQuaid EL, Spirito A, Klein RB. Symptom perception in pediatric asthma: relationship to functional morbidity and psychological factors. J Am Acad Child Adolesc Psychiatry. 1996; 35:1033–1041. PMID: 8755800.

28. Won YK, Hwang TH, Roh EJ, Chung EH. Seasonal patterns of asthma in children and adolescents presenting at emergency departments in Korea. Allergy Asthma Immunol Res. 2016; 8:223–229. PMID: 26922932.

29. Jung CG, Park HS. Factors predicting recovery from asthma exacerbations. Allergy Asthma Immunol Res. 2016; 8:479–480. PMID: 27582397.

30. Rietveld S, Everaerd W. Perceptions of asthma by adolescents at home. Chest. 2000; 117:434–439. PMID: 10669687.

Fig. 1
Graph of the changes in FEV1 according to BHR grade by increasing dose-step. The linear mixed effect analysis showed that the methacholine dose-step affected ΔFEV1%. The BHR grades also affected ΔFEV1%. FEV1, forced expiratory volume in 1 second; BHR, bronchial hyperresponsiveness; ΔFEV1%, percentage fall in the forced expiratory volume in 1 second from baseline.
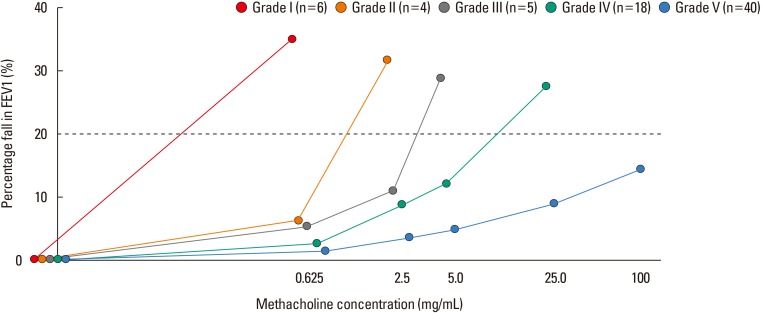
Fig. 2
Distribution of the median mBorg scores in each BHR group during the methacholine provocation test. Except for one point (BHR grade IV at a methacholine concentration of 2.5 mg/mL), the median mBorg score increased with higher dose-steps in all BHR groups. mBorg, modified Borg; BHR, bronchial hyperresponsiveness.
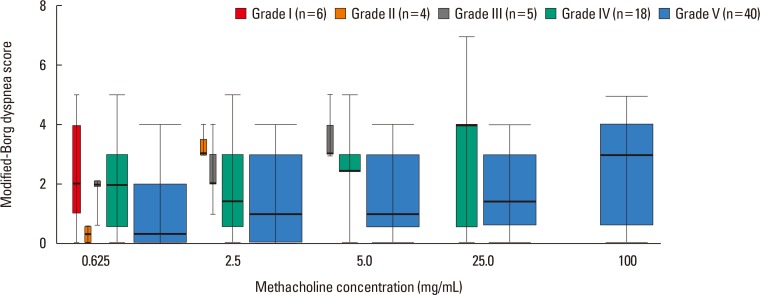
Fig. 3
Distribution of the mean value of ΔFEV1% per each mBorg score according to dose step. A significant interaction was observed between the methacholine dose step and ΔFEV1%. mBorg, modified Borg; ΔFEV1%, percentage fall in the forced expiratory volume in 1 second from baseline; FEV1, forced expiratory volume in 1 second.
Table
Characteristics of the study subjects
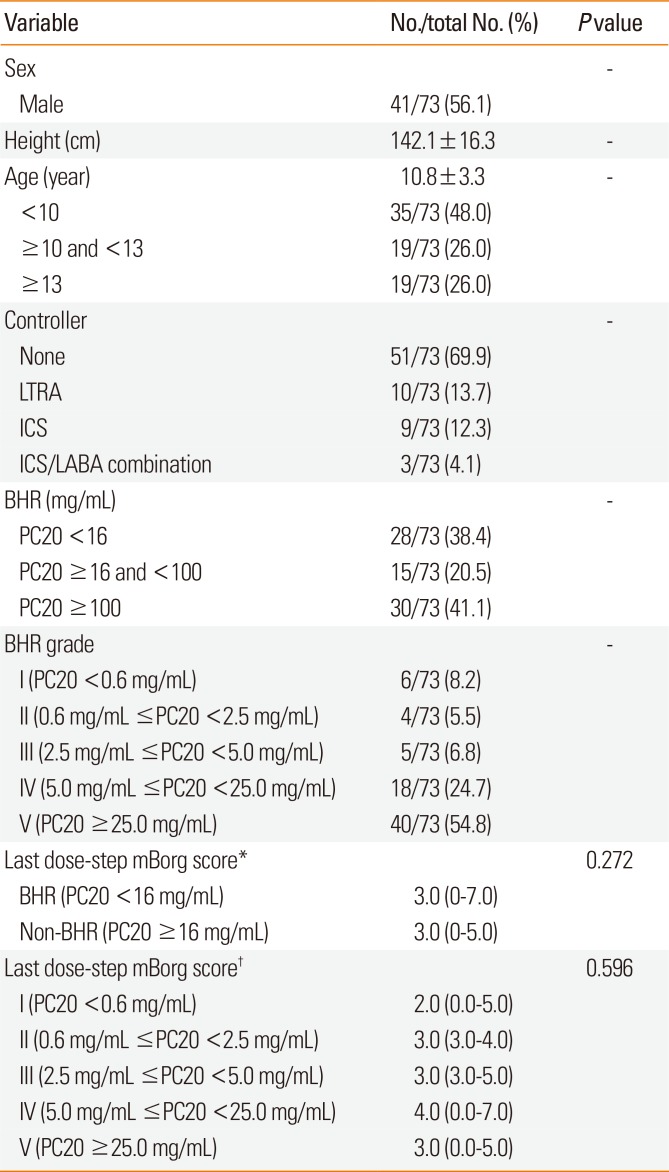
Values are presented as number (%), mean±SD, or median (range).
LTRA, leukotriene receptor antagonist; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; BHR, bronchial hyperresponsiveness; PC20, ΔFEV1% of 20%; mBorg, modified Borg; SD, standard deviation.
*Analysis of difference in scores between subjects who had BHR and those who did not; †Analysis of difference in scores among the 5 BHR groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download