Abstract
Purpose
This report describes the results of a survey of the characteristics of pertussis in children from a single institution and com-pares it to data from the Korea Centers of Disease Control (KCDC).
Methods
We retrospectively evaluated the medical records of 17 and 6 patients diagnosed with pertussis and parapertussis, respectively, at Soonchunhyang University Bucheon Hospital from January 2005 to January 2017.
Results
Of the 17 patients with pertussis, 9 were under 1 year of age (52.9%), 3 were aged between 1 and 10 years (17.6%), and 5 were over 10 years of age (29.4%). Seven patients (41.2%) had never received diphtheria-tetanus-acellular pertussis vaccines, of which 5 were infants below 2 months of age and 2 were 10 years old and lived in China. Four patients showed the initial symptoms of cough in China. The sources of infection were the parents (2 cases) and the siblings (8 cases). All patients showed prolonged severe cough and the average duration of cough was 26 days. Severe symptoms, including dyspnea, cyanosis, apnea, and seizures, were observed in the children under 2 months of age. According to the recent 10-year KCDC data, the highest rate of pertussis diagnosis was noted in infants (47.8%), followed by adolescents (18.7%). Six patients with parapertussis also presented with prolonged severe cough without any other severe symptoms. Lymphocytosis was not found, unlike the patients with pertussis.
Go to : 
REFERENCES
1. Chen Z, Zhang J, Cao L, Zhang N, Zhu J, Ping G, et al. Seroprevalence of pertussis among adults in China where whole cell vaccines have been used for 50 years. J Infect. 2016; 73:38–44.

2. Ibrahim NM, El-Kady EM, Eissa SA, Wahby AF. Assessment of antibody level and avidity against Bordetella pertussis in a cohort of Egyptian indi-viduals aged 1-18 years. J Adv Res. 2016; 7:105–11.

3. Edwards KM. Overview of pertussis: focus on epidemiology, sources of infection, and long term protection after infant vaccination. Pediatr Infect Dis J. 2005; 24(6 Suppl):S104–8.
4. Pimentel AM, Baptista PN, Ximenes RA, Rodrigues LC, Magalhães V. Pert-Pertussis Study Group. Pertussis may be the cause of prolonged cough in adolescents and adults in the interepidemic period. Braz J Infect Dis. 2015; 19:43–6.

5. Teepe J, Broekhuizen BD, Ieven M, Loens K, Huygen K, Kretzschmar M, et al. Prevalence, diagnosis, and disease course of pertussis in adults with acute cough: a prospective, observational study in primary care. Br J Gen Pract. 2015; 65:e662–7.

6. Ewanowich CA, Chui LW, Paranchych MG, Peppler MS, Marusyk RG, Albritton WL. Major outbreak of pertussis in northern Alberta, Canada: analysis of discrepant direct fluorescent-antibody and culture results by using polymerase chain reaction methodology. J Clin Microbiol. 1993; 31:1715–25.

7. Winter K, Harriman K, Zipprich J, Schechter R, Talarico J, Watt J, et al. California pertussis epidemic, 2010. J Pediatr. 2012; 161:1091–6.

8. Munoz FM. Pertussis in infants, children, and adolescents: diagnosis, treatment, and prevention. Semin Pediatr Infect Dis. 2006; 17:14–9.

9. Korea Centers for Disease Control and Prevention. Disease web statistics system [Internet]. Cheongju: Korea Centers for Disease Control and Pre-vention;2016. [cited 2016 Dec 1]. Available from. https://is.cdc.go.kr/dstat/index.jsp.
10. Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008; 9:201–11.

11. Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012; 27:1547–51.

12. Bolding J, Kamat D. Whooping cough caused by bordetella parapertussis. Infect Med. 2004; 21:305–7.
14. Han YI, Choi JY, Lee H, Lee TJ. Active surveillance of pertussis in infants under 6 months of age: a single center experience from 2011 to 2013. Korean J Pediatr Infect Dis. 2014; 21:114–20.

15. Tsang RS, Lau AK, Sill ML, Halperin SA, Van Caeseele P, Jamieson F, et al. Polymorphisms of the fimbria fim3 gene of Bordetella pertussis strains isolated in Canada. J Clin Microbiol. 2004; 42:5364–7.
16. Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conser-vation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microbiol. 2004; 53(Pt 5):355–65.

17. Mastrantonio P, Spigaglia P, van Oirschot H, van der Heide HG, Heuvel-man K, Stefanelli P, et al. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology. 1999; 145(Pt 8):2069–75.

18. Kim SH, Lee J, Sung HY, Yu JY, Kim SH, Park MS, et al. Recent trends of antigenic variation in Bordetella pertussis isolates in Korea. J Korean Med Sci. 2014; 29:328–33.
19. Yoo S, Ahn KO, Park EH, Cho HS, Park CY, Lee HR. Epidemiologic and clinical deatures of pertussis in children (2000.3-2001.3). J Korean Pediatr Soc. 2002; 45:603–8.
20. Lee SY, Han SB, Kang JH, Kim JS. Pertussis prevalence in Korean adolescents and adults with persistent cough. J Korean Med Sci. 2015; 30:988–90.

21. Lee SY, Choi UY, Kim JS, Ahn JH, Choi JH, Ma SH, et al. Immunoassay of pertussis according to ages. Korean J Pediatr Infect Dis. 2012; 19:55–60.

22. Rothstein E, Edwards K. Health burden of pertussis in adolescents and adults. Pediatr Infect Dis J. 2005; 24(5 Suppl):S44–7.

23. Forsyth K, Tan T, von König CH, Caro JJ, Plotkin S. Potential strategies to reduce the burden of pertussis. Pediatr Infect Dis J. 2005; 24(5 Suppl):S69–74.

24. Van Buynder PG, Owen D, Vurdien JE, Andrews NJ, Matthews RC, Miller E. Bordetella pertussis surveillance in England and Wales: 1995-7. Epidemiol Infect. 1999; 123:403–11.

25. Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J. Defining pertussis epidemiology: clinical, microbiologic and serologic perspectives. Pediatr Infect Dis J. 2005; 24(5 Suppl):S25–34.
26. Rendi-Wagner P, Tobias J, Moerman L, Goren S, Bassal R, Green M, et al. The seroepidemiology of Bordetella pertussis in Israel–Estimate of incidence of infection. Vaccine. 2010; 28:3285–90.

27. Kim MR, Kang HJ, Lee HJ. Diagnosis of bordetella pertussis infections by polymerase chain reaction. J Korean Pediatr Soc. 1996; 39:1260–70.
28. Riffelmann M, Wirsing von König CH, Caro V, Guiso N. Pertussis PCR Consesus Group. Nucleic Acid amplification tests for diagnosis of Bordetella infections. J Clin Microbiol. 2005; 43:4925–9.
29. Heininger U, Kleemann WJ, Cherry JD. Sudden Infant Death Syndrome Study Group. A controlled study of the relationship between Bordetella pertussis infections and sudden unexpected deaths among German infants. Pediatrics. 2004; 114:e9–15.

30. Halasa NB, Barr FE, Johnson JE, Edwards KM. Fatal pulmonary hyper-tension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics. 2003; 112(6 Pt 1):1274–8.

31. Ntezayabo B, De Serres G, Duval B. Pertussis resurgence in Canada large-ly caused by a cohort effect. Pediatr Infect Dis J. 2003; 22:22–7.

32. Granström M, Granström G, Gillenius P, Askelöf P. Neutralizing antibodies to pertussis toxin in whooping cough. J Infect Dis. 1985; 151:646–9.
Go to : 
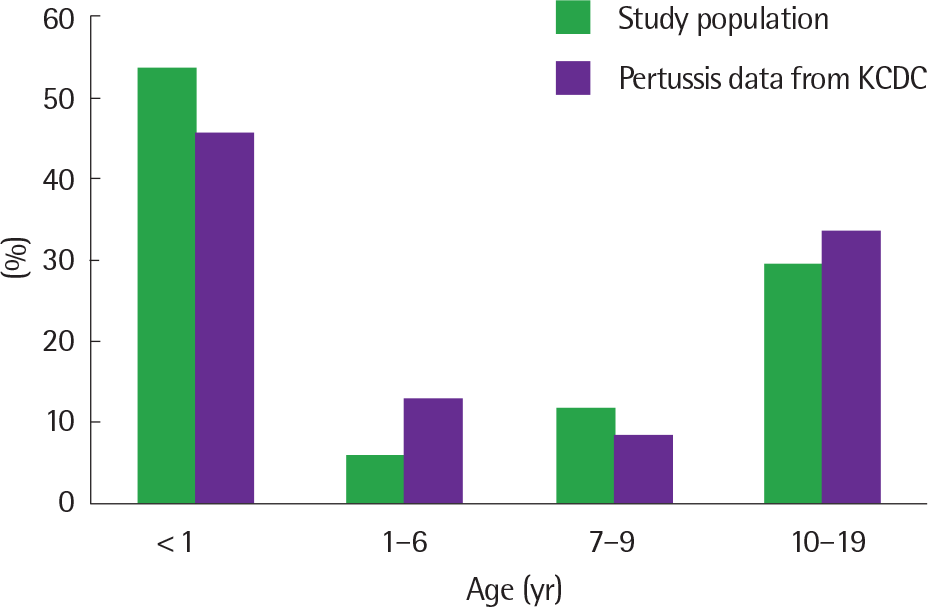 | Fig. 1.Age distribution of pertussis: comparison to Korea Centers for Disease Control and Prevention (KCDC). |
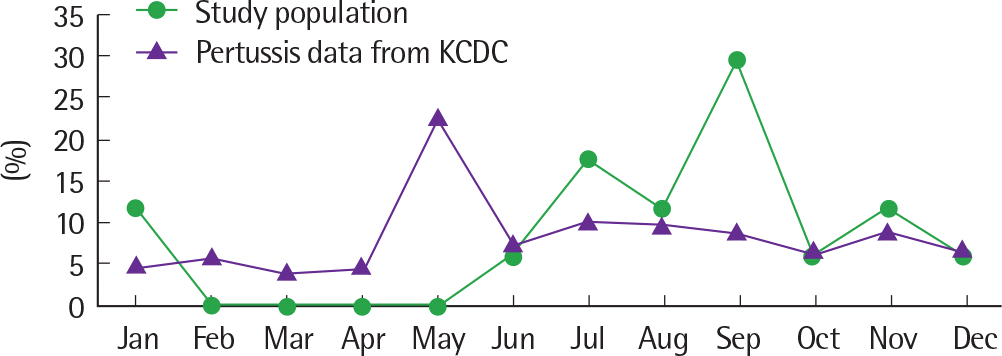 | Fig. 2.Monthly incidence of pertussis: comparison to Korea Centers for Disease Control and Prevention (KCDC). |
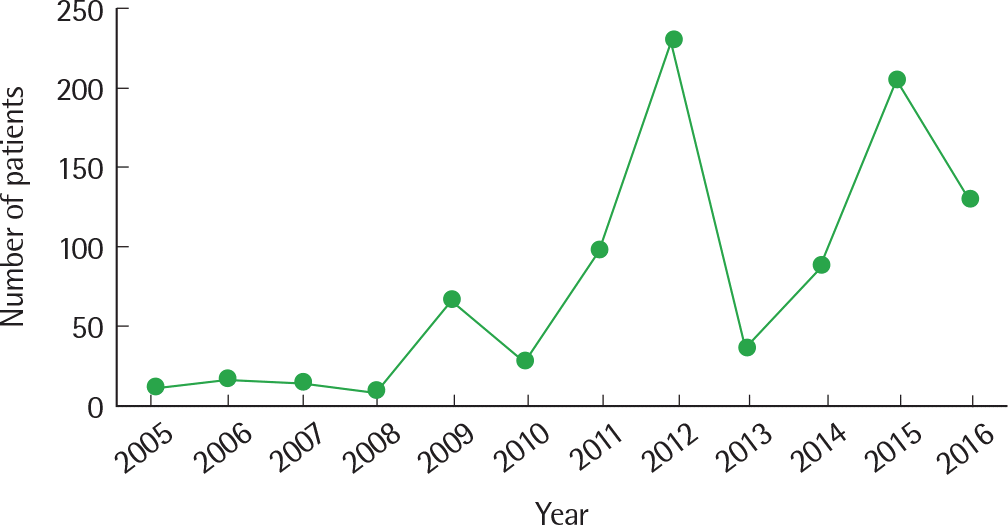 | Fig. 3.Pertussis incidence from 2005 to 2016 (Korea Centers for Disease Control and Prevention data. |
Table 1.
Clinical characteristics of pertussis according to age
| Variable | Total (n=17) | Age<1 yr (n=9) | Age≥1 yr (n=8) | P-value∗ |
|---|---|---|---|---|
| Disease duration (day) | 26.2±9.0 | 29.1±8.8 | 23.0±8.6 | 0.161 |
| Symptom | ||||
| Cough | 17 (100) | 9 (100) | 8 (100) | NA |
| Sputum | 8 (47.1) | 4 (44.4) | 4 (50.0) | 1.000 |
| Rhinorrhea | 9 (52.9) | 5 (55.6) | 4 (50.0) | 1.000 |
| Fever | 2 (11.8) | 1 (11.1) | 1 (12.5) | 1.000 |
| Vomiting | 4 (23.5) | 4 (44.4) | 0 (0) | 0.113 |
| Cyanosis | 5 (29.4) | 5 (55.6) | 0 (0) | 0.048 |
| Dyspnea | 5 (29.4) | 5 (55.6) | 0 (0) | 0.048 |
| Apnea | 2 (11.8) | 2 (22.2) | 0 (0) | 0.506 |
| Convulsion | 2 (11.8) | 2 (22.2) | 0 (0) | 0.506 |
| Severity | 0.001 | |||
| Severe† | 8 (47.1) | 8 (88.9) | 0 (0) | |
| Mild‡ | 9 (52.9) | 1 (11.1) | 8 (100) | |
| Infiltration on the chest x-ray | 0.661 | |||
| No | 13 (76.5) | 6 (66.7) | 7 (87.5) | |
| Yes | 4 (23.5) | 3 (33.3) | 1 (12.5) | |
| WBC (/mm3) | 16,494.2±6,690.7 | 17,961.1±6,035.1 | 12,093.3±7,860.3 | 0.166 |
| Lymphocyte (%) | 61.5±19.1 | 69.8±9.9 | 36.8±19.8 | 0.033 |
| Absolute No. of lymphocyte | 10,560.7±5,884.8 | 12,767.1±4,938.4 | 3,941.3±2,299.6 | 0.033 |
Table 2.
Differences in clinical symptoms, laboratory results, and chest radiograph findings in pertussis according to the number of DTaP vaccinations
| Variable | No injection (n=7) | 1-4 Injections (n=5) | 5 Injections (n=5) | P-value∗ |
|---|---|---|---|---|
| Age (yr) | 3.3±5.6 | 0.9±1.7 | 10.2±3.3 | 0.032 |
| Disease duration (day) | 26.0±9.7 | 29.0±8.5 | 23.8±9.7 | 0.697 |
| Symptom | ||||
| Cough | 7 (100) | 5 (100) | 5 (100) | NA |
| Sputum | 5 (71.4) | 1 (20.0) | 2 (40.0) | 0.198 |
| Rhinorrhea | 3 (42.9) | 3 (60.0) | 3 (60.0) | 0.784 |
| Fever | 0 (0) | 1 (20.0) | 1 (20.0) | 0.452 |
| Vomiting | 1 (14.3) | 3 (60.0) | 0 (0) | 0.062 |
| Cyanosis | 2 (28.6) | 3 (60.0) | 0 (0) | 0.114 |
| Dyspnea | 4 (57.1) | 1 (20.0) | 0 (0) | 0.087 |
| Apnea | 2 (28.6) | 0 (0) | 0 (0) | 0.198 |
| Convulsion | 2 (28.6) | 0 (0) | 0 (0) | 0.198 |
| Severity | 0.040 | |||
| Severe† | 5 (71.4) | 3 (60.0) | 0 (0.0) | |
| Mild‡ | 2 (28.6) | 2 (40.0) | 5 (100) | |
| Infiltration on the chest x-ray | 0.571 | |||
| No | 6 (85.7) | 3 (60.0) | 4 (80.0) | |
| Yes | 1 (14.3) | 2 (40.0) | 1 (20.0) | |
| WBC (/mm3) | 14,788.3±7,546.4 | 19,662.5±5,299.7 | 15,275.0±7,926.7 | 0.397 |
| Lymphocyte (%) | 68.6±9.8 | 68.8±11.8 | 25.8±7.4 | 0.099 |
| Absolute No. of lymphocyte | 10,407.0±5,618.0 | 13,957.8±5,370.4 | 4,227.5±3,175.6 | 0.174 |
Table 3.
Comparison between clinical symptoms and laboratory results of pertussis and parapertussis
| Variable | Pertussis (n=17) | Parapertussis (n=6) | P-value∗ |
|---|---|---|---|
| Disease duration (day) | 26.2±9.0 | 18.8±7.1 | 0.131 |
| Symptom | |||
| Cough | 17 (100) | 6 (100) | NA |
| Sputum | 8 (47.1) | 3 (50.0) | 1.000 |
| Rhinorrhea | 9 (52.9) | 1 (16.7) | 0.288 |
| Fever | 2 (11.8) | 3 (50.0) | 0.169 |
| Vomiting | 4 (23.5) | 0 (0) | 0.496 |
| Cyanosis | 5 (29.4) | 0 (0) | 0.354 |
| Dyspnea | 5 (29.4) | 0 (0) | 0.354 |
| Apnea | 2 (11.8) | 0 (0) | 0.971 |
| Convulsion | 2 (11.8) | 0 (0) | 0.971 |
| Severity | 0.114 | ||
| Severe† | 8 (47.1) | 0 (0) | |
| Mild‡ | 9 (52.9) | 6 (100) | |
| Infiltration on the chest x-ray | 1.000 | ||
| No | 13 (76.5) | 4 (66.7) | |
| Yes | 4 (23.5) | 2 (33.3) | |
| WBC (/mm3) | 16,494.2±6,690.7 | 6,663.3±2,532.2 | 2 0.021 |
| Lymphocyte (%) | 61.5±19.1 | 44.5±8.2 | 0.112 |
| Absolute No. of lymphocyte | 10,560.7±5,884.8 | 2,831.6±642.7 | 0.030 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download