Abstract
Purpose
Steroids can be used as an adjuvant therapy in the management of mycoplasma pneumonia, but no definite guidelines for the use of steroids have been established. The purpose of this study was to analyze the current usage and effects of steroids in the management of childhood mycoplasma pneumonia in a secondary hospital in Korea.
Methods
We retrospectively reviewed the medical records of 152 patients who were admitted due to mycoplasma pneumonia. The patients were divided into 3 groups as follows: those who did not use steroids (81 patients, 53%), those who used steroids after their fever subsided (42 patients, 28%) and those who used steroids during fever (29 patients, 19%).
Results
In decreasing order of values, the duration of fever during hospitalization (60.0±40.2 hours vs. 37.3±28.5 hours vs. 29.7±29.5 hours, P=0.006) and duration of hospitalization (5.9±1.7 days vs. 5.0±1.4 days vs. 4.0±1.5 days, P<0.001) were reported in the group which received steroids during fever, the group which received steroids after the fever subsided and the group which did not receive steroids. In the group which received steroids during fever, patients with early steroid use (within 24 hours) had a shorter fever duration in the hospital (12.0 hours vs. 73.5 hours, P <0.001) and a hospitalization duration (5.0 days vs. 6.5 days, P=0.007) than those with late steroid use (after 24 hours).
REFERENCES
1. Yu J. Clinical issues regarding increased macrolide-resistant Mycoplasma pneumoniae in children. Allergy Asthma Respir Dis. 2017; 5:1–2.
2. Kim JH, Kim JY, Yoo CH, Seo WH, Yoo Y, Song DJ, et al. Macrolide resistance and its impacts on M. pneumoniae pneumonia in children: comparison of two recent epidemics in Korea. Allergy Asthma Immunol Res. 2017; 9:340–6.

3. Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. 2015; 58:172–7.
4. Kim JH, Chae SA, Lee DK. Clinical findings of Mycoplasma pneumonia in children, from 1998 to 2003. Korean J Pediatr. 2005; 48:969–75.
5. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008; 56:326–31.

6. He J, Liu M, Ye Z, Tan T, Liu X, You X, et al. Insights into the pathogenesis of Mycoplasma pneumoniae (Review). Mol Med Rep. 2016; 14:4030–36.
7. Balish MF, Santurri RT, Ricci AM, Lee KK, Krause DC. Localization of Mycoplasma pneumoniae cytadherence-associated protein HMW2 by fusion with green fluorescent protein: implications for attachment organ-elle structure. Mol Microbiol. 2003; 47:49–60.
8. Prince OA, Krunkosky TM, Krause DC. In vitro spatial and temporal analysis of Mycoplasma pneumoniae colonization of human airway epi-thelium. Infect Immun. 2014; 82:579–86.
9. Großhennig S, Schmidl SR, Schmeisky G, Busse J, Stülke J. Implication of glycerol and phospholipid transporters in Mycoplasma pneumoniae growth and virulence. Infect Immun. 2013; 81:896–904.

10. Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009; 4:e7562.

11. Lynch M, Taylor TK, Duignan PJ, Swingler J, Marenda M, Arnould JP, et al. Mycoplasmas in Australian fur seals (Arctocephalus pusillus dorifer-us): identification and association with abortion. J Vet Diagn Invest. 2011; 23:1123–30.

12. Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis. 2013; 19:1281–4.
13. Xin D, Mi Z, Han X, Qin L, Li J, Wei T, et al. Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother. 2009; 53:2158–9.
14. Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2008; 52:348–50.
15. Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. 2017; 60:151–7.

16. Jackson MA, Schutze GE. Committee on infectious diseases. The use of systemic and topical fluoroquinolones. Pediatrics. 2016; 138(5):pii: e20162706.

17. Youn YS, Lee KY. Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2012; 55:42–7.
18. Busson L, Van den Wijngaert S, Dahma H, Decolvenaer M, Di Cesare L, Martin A, et al. Evaluation of 10 serological assays for diagnosing Mycoplasma pneumoniae infection. Diagn Microbiol Infect Dis. 2013; 76:133–7.

19. You SY, Jwa HJ, Yang EA, Kil HR, Lee JH. Effects of methylprednisolone pulse therapy on refractory Mycoplasma pneumoniae pneumonia in children. Allergy Asthma Immunol Res. 2014; 6:22–6.
20. Shin JE, Cheon BR, Shim JW, Kim DS, Jung HL, Park MS, et al. Increased risk of refractory Mycoplasma pneumoniae pneumonia in children with atopic sensitization and asthma. Korean J Pediatr. 2014; 57:271–7.
21. Oh JW. The efficacy of glucocorticoid on macrolide resistant Mycoplasma pneumonia in children. Allergy Asthma Immunol Res. 2014; 6:3–5.

22. Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2006; 41:263–8.
23. Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, et al. Effects of prednisolone on refractory mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2014; 49:377–80.

24. Seo YH, Kim JS, Seo SC, Seo WH, Yoo Y, Song DJ, et al. Predictive value of C-reactive protein in response to macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean J Pediatr. 2014; 57:186–92.
Fig. 1.
Comparison of fever duration (hr) after admission in 2 groups according to steroid start time (within 24 hours vs. after 24 hours).
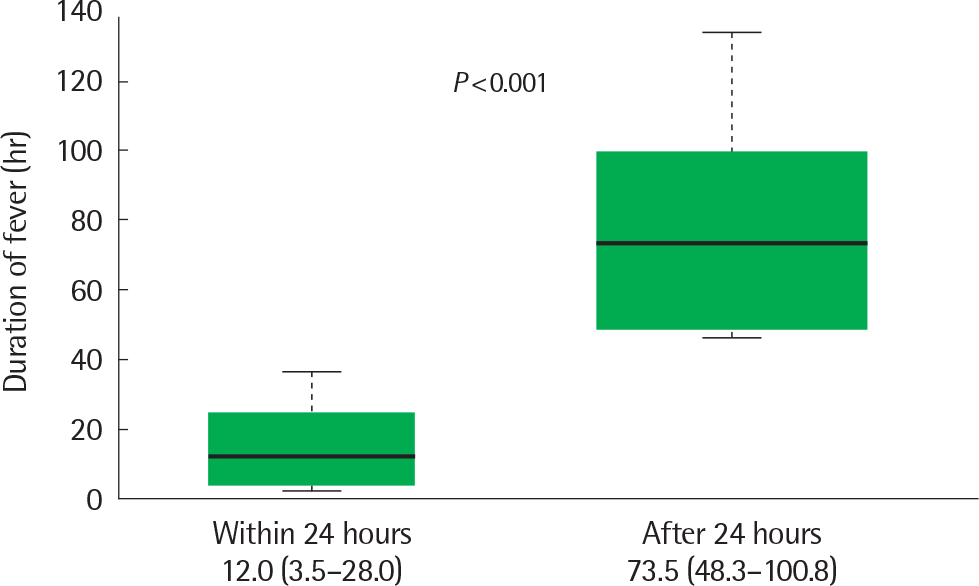
Fig. 2.
Comparison of duration of hospitalization (day) in 2 groups according to steroid start time (within 24 hours vs. after 24 hours).
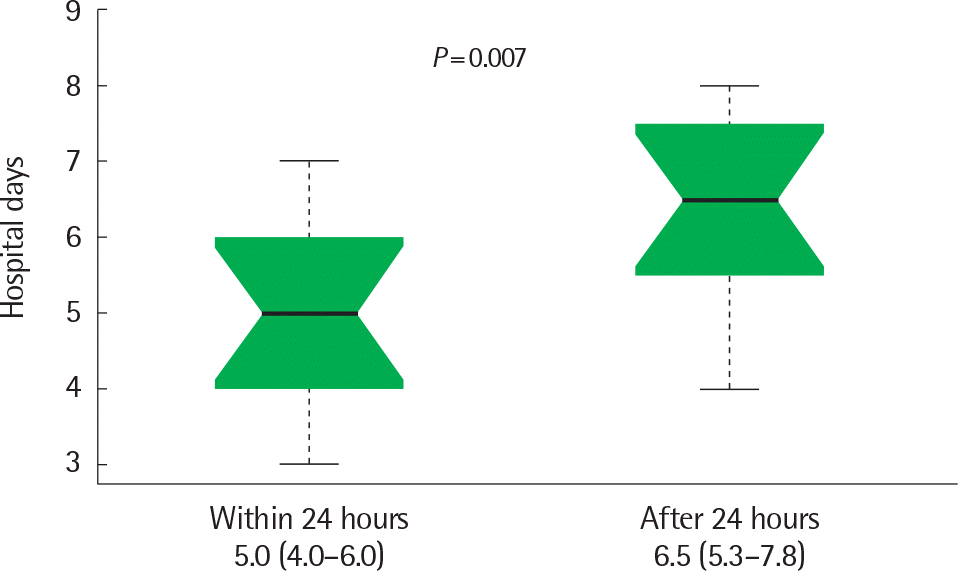
Table 1.
Comparison of demographic data, duration of fever and hospitalization in each group of subjects
| Variable | Group I | Group II | Group III | P-value |
|---|---|---|---|---|
| No. of patients | 81 (53) | 42 (28) | 29 (19) | |
| Age (yr) | 4.7±3.0 | 4.5±2.5 | 4.3±2.8 | 0.804 |
| Sex, male:female | 33:48 | 23:19 | 16:13 | 0.217 |
| Fever duration (day) | ||||
| Before admission | 4.1±2.3 | 4.3±1.9 | 3.8±2.0 | 0.638 |
| After admission | 29.7±29.5 | 37.3±28.5 | 60.0±40.2 | 0.006∗ |
| Duration of hospitalization (day) | 4.0±1.5 | 5.0±1.4 | 5.9±1.7 | <0.001∗ |
| Rash | 2 (1.2) | 0 (0) | 1 (3.4) | 0.529 |
| Vomiting | 10 (12.3) | 6 (14.3) | 7 (24.1) | 0.310 |
| Diarrhea | 8 (9.9) | 7 (16.7) | 5 (17.2) | 0.441 |
Table 2.
Comparison of type of pneumonia and treatment in each group of subjects
| Variable | Group I | Group II | Group III | P-value |
|---|---|---|---|---|
| Type | 0.004∗ | |||
| Bronchopneumonia | 77 (95.0) | 36 (85.7) | 21 (72.4) | |
| Lobar pneumonia | 4 (4.9) | 6 (14.2) | 8 (27.5) | |
| Macrolide | 0.083 | |||
| Azithromycin | 31 (40.7) | 23 (57.5) | 18 (72.0) | |
| Roxithromycin | 38 (50.0) | 13 (32.5) | 4 (16) | |
| Clarithromycin | 7 (9.2) | 3 (7.5) | 3 (12) | |
| Levofloxacin | - | 1 (2.5) | - | |
| Steroid dose (mg/kg) | - | 1.2±0.2 | 1.2±0.2 | 0.930 |
| Steroid duration (day) | - | 3.5±1.0 | 4.8±1.1 | <0.001∗ |
Table 3.
Comparison of blood test results in each group of subjects
| Variable | Group I | Group II | Group III | P-value |
|---|---|---|---|---|
| WBC (×103/μL) | 9.63±4.52 | 7.99±2.61 | 8.45±3.45 | 0.066 |
| Hb (g/dL) | 12.3±0.8 | 12.1±1.0 | 11.9±0.8 | 0.130 |
| Neutrophil counts (×103/μL) | 5.94±3.57 | 4.65±1.52 | 4.33±1.74 | 0.009∗ |
| Neutrophils (%) | 59.8±13.8 | 57.8±14.3 | 52.5±12.4 | 0.050 |
| ESR (mm/hr) | 28.7±17.0 | 28.2±12.8 | 22.7±12.3 | 0.174 |
| CRP (mg/L) | 24.1±26.1 | 22.9±17.5 | 20.4±18.3 | 0.757 |
| LDH (IU/L) | 306.0±64.0 | 343.0±56.0 | 363.0±92.0 | 0.001∗ |
| AST (IU/L) | 32.4±8.7 | 39.9±17.8 | 39.1±9.9 | 0.002∗ |
| ALT (IU/L) | 14.2±5.0 | 21.8±30.4 | 15.6±4.9 | 0.051 |
Group I, treatment without steroids; group II, steroid use after fever subside; group III, steroid use with fever; WBC, white blood cells; Hb, hemoglobin; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 4.
Comparison of demographic data in 2 groups according to steroid start time
| Variable | Steroid use | P-value | |
|---|---|---|---|
| Within 24 hr (n=13) | After 24 hr (n=16) | ||
| Age (yr) | 2.9 (1.7–5.7) | 5.2 (2.3–5.8) | 0.503 |
| Sex, male:female | 7:6 | 9:7 | |
| Fever duration before admission (day) | 3.0 (1.0, 4.0) | 5.0 (3.3, 5.8) | 0.015∗ |
| Type | 0.031∗ | ||
| Bronchopneumonia | 12 (92.3) | 9 (56.3) | |
| Lobar pneumonia | 1 (7.7) | 7 (43.7) | |
Table 5.
Comparison of blood test results in two groups according to steroid start time




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download