Abstract
The Endeavor Resolute® (ER) is a zotarolimus-eluting stent (ZES) with a biocompatible BioLinx polymer. This study prospectively compared the clinical outcomes of 2 versions of ZES, ER and Endeavor Sprint® (ES), in patients with multivessel disease. A total of 488 patients who underwent multivessel percutaneous coronary intervention (PCI) were divided into 2 groups the ER group (n=288) and the ES group (n=200). The primary endpoint was a composite of major adverse cardiac events (MACE) consisting of death, myocardial infarction, and target vessel revascularization after 12 months. In all patients, the prevalence of diabetes was higher in the ER group (42.7% vs. 31.0%, p=0.009). The rate of post-PCI Thrombolysis in Myocardial Infarction flow grade 3 was higher in the ER group (100.0% vs. 98.0%, p=0.028). There were no between-group differences in the in-hospital, 1-month and 12-month clinical outcomes. In the propensity score matched cohort (n=200 in each group), no differences were observed in the baseline and procedural characteristics. There were no statistical differences in the rates of in-hospital, 1-month and 12-month events (12-month MACE in the ER and ES groups: 6.0% vs. 3.5%, p=0.240, respectively). The safety and efficacy of both versions of ZES were comparable in patients with multivessel disease during a 12-month clinical follow-up.
Drug-eluting stents (DES) reduce the coronary restenosis rate after stenting by inhibiting neointimal growth of smooth muscle cells. Zotarolimus is a synthesized rapamycin analogue with both anti-proliferative and anti-inflammatory effects.1 Endeavor Sprint® (ES) (Medtronic, MN, USA), the first zotarolimus-eluting stent (ZES), used a polymer mimicking the cell membrane phospholipid phosphorylcholine. Zotarolimus is eluted mostly (95%) within 14 days. Endeavor Resolute® (ER) (Medtronic, MN, USA), the second ZES, had a biocompatible BioLinx polymer and extended delivery of zotarolimus with 85% of drug released within 60 days and the remainder by 180 days.2 More recently introduced ZESs include the Resolute Integrity® with greater deliverability and conformability and the Resolute Onyx® with improved visibility, a low crossing profile, and an enhanced delivery system.34
ZES has shown good clinical outcomes256789101112 but currently there is a paucity of clinical data comparing the ER and the ES.13 In the present study we sought to evaluate the overall clinical performance and the safety of ER in comparison with ES in patients with multivessel coronary artery disease.
HEART (Honam EndeAvor ResoluTe) Trial is a prospective, multicenter observational study comparing the clinical benefits of ER and ES in patients with multivessel coronary artery disease in Korea. Between April 2009 and May 2011, a total of 488 patients were enrolled who underwent multivessel percutaneous coronary intervention (PCI) at 9 academic and community-based institutions in the southwestern region of Korea. The present study was conducted according to the Declaration of Helsinki. The institutional review board of all participating centers approved the study protocol. The approval number was E-2009-05-048 at Chonnam National University Hospital. Written informed consent was obtained from all participating patients.
Patients with multivessel coronary artery disease (at least 2 vessels) who underwent multivessel stent implantation with ER or ES (stent diameter: 2.5–4.0 mm, stent length: 8/9–30 mm) were deemed eligible for the trial. Inclusion criteria were patients aged ≥18 years, patients with coronary artery disease (CAD) who needed to be treated in at least 2 coronary vessels. CAD included stable angina pectoris and acute coronary syndrome [unstable angina pectoris, non-ST elevation and ST-elevation myocardial infarction (MI)]. Patients with chronic total occlusion were also deemed eligible. All patients consented to participate and authorized the collection and release of their medical information by signing the “Patient Informed Consent Form.” All lesions requiring interventions in 2 or more native coronary arteries were amendable for the implantation of 2 or more ZES. The patient or guardian was willing and able to cooperate with the study procedures and required follow-up visits. Exclusion criteria were patients with left main stem disease, patients with hypersensitivity or allergies to drugs or components in use with PCI, history of bleeding diathesis or known coagulopathy, gastrointestinal or genitourinary bleeding within the prior 3 months, major surgery within 2 months, platelet count <150,000 cells/mm3 or hemoglobin <9 g/dl, previous coronary intervention on target vessel or graft vessel disease, transplant patients, patients with left ventricular ejection fraction <30%, patients with cardiogenic shock, patients with a life expectancy <12 months, patients with kidney dysfunction (serum creatinine >2.0 mg/dl or dependence on dialysis), patients with severe hepatic dysfunction (AST and ALT >3 times upper normal reference values) and patients with known malignancy.
After PCI, aspirin at least 75 mg daily was given indefinitely. Clopidogrel 75 mg daily was given for at least 6 months. The participating centers followed the same anti-platelet regimen in terms of the duration for both study arms.
Diabetes was defined as a history of diabetes, regardless of duration of disease, need for antidiabetic agents, or a fasting blood glucose >126 mg/dl. Hypertension was defined as a history of hypertension diagnosed and treated with medication, diet and/or exercise, or blood pressure >140 mmHg systolic or 90 mmHg diastolic on at least 2 occasions, or currently being on antihypertensive pharmacologic therapy. Dyslipidemia was defined as total cholesterol >200 mg/dl, or low-density lipoprotein cholesterol ≥130 mg/dl, or high-density lipoprotein cholesterol <30 mg/dl, or admission cholesterol >200 mg/dl, or triglycerides >150 mg/dl. A family history of CAD was indicated if the patient had any direct blood relatives (parents, siblings, children) who had any of the following diagnosed at age <55 years: angina, previous coronary artery bypass graft surgery (CABG) or PCI, or MI, or sudden cardiac death without obvious cause. Chronic lung disease was defined as a history of chronic bronchitis or a diagnosis of moderate or severe obstructive (forced expiratory volume in one second <70% of predicted) or restrictive (vital capacity <70% of predicted) syndrome on spirometry.
The primary clinical endpoint was major adverse cardiac events (MACE) at 12 months. MACE were defined as the composite of death from any cause, new MI, ischemia-driven target vessel revascularization (TVR) by either PCI or CABG. Secondary end points included stent thrombosis and individual components of MACE at 12 months. TVR was considered ischemia-driven if associated with a positive functional study, a target vessel diameter stenosis ≥50% by core laboratory quantitative analysis with ischemic symptoms. The target vessel was defined as the entire major coronary vessel proximal and distal to the target lesion, which included upstream and downstream branches and the target lesion itself. MI was defined as either the development of new pathological Q waves ≥0.4 seconds in duration in ≥2 contiguous leads or an elevation of cardiac biomarkers (positive troponin-I or T, or creatine phosphokinase levels to >2.0 times normal with positive creatine phosphokinase-MB). Stent thrombosis was defined according to the Academic Research Consortium definitions.14
Clinical follow-up was done by phone or preferably by outpatient clinic visit. Follow-up coronary angiography was performed on an ischemic-driven basis. For each patient, a Case Report Form (CRF) for data recording was provided. CRFs were numbered and used in ascending numerical order. All data were recorded in a dedicated database. The investigator ensured that patient anonymity was maintained. On CRFs or other documents, patients were not identified by their names but by their CRF code. The investigator kept a separate log of patient codes, names, and addresses.
Differences between the group means were assessed with the 2-tailed Student t test. The Chi-Square test or Fisher's exact test was used to test the differences between proportions. Hazard ratios (HR) and their 95% confidence intervals (CI) were calculated for the outcome variables using Cox regression analysis. To adjust for the bias inherent to the decision of choosing ER versus ES, propensity scores were used.1516 The propensity scores were estimated for the likelihood of receiving ER using a multiple logistic regression model that contained 30 covariates shown in Tables 1 and 2: age, sex, smoking, hypertension, diabetes mellitus, dyslipidemia, prior history of MI, heart failure, family history of coronary artery disease, stroke, chronic kidney disease, chronic lung disease, clinical diagnosis, left ventricular ejection fraction, 3-vessel disease, type B2/C lesion, bifurcation lesion, pre-PCI Thrombolysis in Myocardial Infarction (TIMI) flow grade 3, transradial PCI, intravascular ultrasound-guided PCI, staged PCI, and use of aspirin, clopidogrel, glycoprotein IIb/IIIa inhibitor, unfractionated heparin, low molecular weight heparin, beta blocker, calcium channel blocker, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and statin during hospitalization. The c-statistic for the propensity model was 0.68, indicating fair ability to discriminate treatment groups. The Hosmer-Lemeshow goodness-of-fit test p-value was 0.81, confirming good calibration and fit of the multivariable model that estimated the propensity score. Matching was performed using a greedy matching protocol (1:1 nearest neighbor matching without replacement) with a caliper width of 0.2 of the standard deviation.1718 We were able to match 200 patients receiving ER to 200 patients undergoing ES implantation. We estimated standardized differences for all the covariates before and after matching to assess the balance of the covariates between the ER and ES groups. After matching, none of the covariates showed a standardized difference exceeding 10%, suggesting that all of the measured covariates were well balanced between the matched groups.1920 Differences between the matched pairs were evaluated using the paired t test or the Wilcoxon signed rank test for continuous variables and the McNemar's test for categorical variables. The risks of clinical time-to-event endpoints in the matched cohort were compared by using a Cox proportional hazards regression model stratified on matched pairs, including factors deemed significant (p-value<0.1) by univariate analysis or considered clinically important in the multivariate model. We further tested the impact of ER versus ES on 12-month clinical outcome in multiple subgroups of age, sex, diabetes, hypertension, clinical diagnosis, and 3-vessel disease. For subgroup analysis, we repeated the same propensity score matching process while matching on both the score and the subgroup variable, forcing exact matches on the subgroup characteristics. Conditional logistic regression was then used to identify treatment-subgroup interactions. Statistical significance was considered as a 2-tailed p-value<0.05. Statistical analyses were conducted using SPSS version 21 (SPSS Inc., Chicago, IL, USA) and R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
In all patients, the prevalence of diabetes mellitus was higher in the ER group compared to the ES group: 42.7% vs. 31.0%, p=0.009 (Table 1). Post-PCI TIMI flow grade 3 was higher in the ER group than in the ES group: 100.0% vs. 98.0%, p=0.028 (Table 2). Medical treatment during hospitalization including dual antiplatelet therapy was not different between the groups (Table 2). In the propensity-matched cohort, there were no significant differences in baseline clinical and procedural characteristics and in-hospital medical treatment (Tables 1, 2). The rates of dual antiplatelet therapy at 12 months in ER and ES groups were 80.6% and 81.0% in all patients (p=0.903) and 79.0% and 81.0% in the propensity-matched cohort (p=0.617).
In all patients, clinical outcomes were not different between the 2 groups during hospitalization, at 1 month and 12 months (Table 3). In the propensity matched cohort, there were no differences in the in-hospital and 1-month events. Twelve-month mortality and MACE were not different between ER and ES groups: 1.0% vs. 1.0% and 5.5% vs. 2.5%, respectively (Table 3, Fig. 1). Subgroup analysis in the propensity-matched cohort showed that the treatment effects of ER and ES for 12-month MACE were similar across all the subgroups (Fig. 2).
This prospective, multicenter clinical study showed that the 2 versions of ZES—ES and ER—are comparable at 12 months in patients with multivessel coronary artery disease. To the best of our knowledge, this is the first clinical trial directly comparing outcomes of ES and ER in multivessel disease.
The 2 versions of ZES—the ES and ER—have the same stent platforms (cobalt alloy with modular cell design), stent delivery system (rapid exchange system). They contain, however, different polymers. ES adopts a phosphorylcholine (PC) polymer, composed of biocompatible materials to mimic biochemical reaction. The PC coated surface does not activate coagulation pathways, increasing hemocompatibility of the polymer. The PC polymer mimics lipid headgroup components of the natural cell membrane of red blood cells, creating a non-active biological interface. It provides very bio-stable layers to the stent surface with a low coefficient of friction. In in-vitro testing, it showed low platelet adhesion and activation. In addition, the PC polymer-coated surface reduced thrombus formation and inflammatory reactions in in-vivo animal tests.212223 Zotarolimus is rapidly eluted from the polymer with 95% of drug released within 14 days. ER adopts BioLinx polymer, a unique blend of 3 polymers, a hydrophilic C19 polymer, a water soluble polyvinyl pyrrolidinone (PVP) and a hydrophobic C10 polymer.24 Like the PC polymer, it is designed to mimic the biological chemistry of the body. The hydrophilic PVP polymer in BioLinx provides good biocompatibility in in-vitro tests such as monocyte adhesion.25 ER elutes 85% of zotarolimus during the first 60 days and the remainder by 180 days.2
ES showed good results in clinical trials. In ENDEAVOR III (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] the Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions) trial, compared with the sirolimus-eluting stent (SES), the clinically driven TLR did not significantly vary between ES and SES (6.3% with Endeavor stent vs. 3.5% with SES, p=0.34) at 9-month follow-up. There were no significant differences between ES and SES in MACE (7.6% vs. 7.1%, p =1.0) and target vessel failure (TVF: cardiac death, MI, or TVR) (12.0% vs. 11.5%, p=1.0). Event-free survival at 9 months for clinically driven TLR, MACE, and TVF did not significantly differ between the ES and SES groups.5 However, by 3 years, ES was associated with a significantly lower rate of death or MI (3.9% vs. 10.8%, p=0.028).6 In the SORT OUT (Danish Organization for Clinical Trials with Clinical Outcome) III trial comparing ES and SES, a composite of cardiac death, MI, and TVR, occurred significantly more often in ES than SES at 9 months (6% vs. 3%, p=0.0002), 18 months (10% vs. 5%, p<0.0001), and 36 months (12.9% vs. 10.1%, p=0.022).78 However, the overall rate of stent thrombosis was similar at 3 years (1.1% vs. 1.4%) and very late stent thrombosis incidence was lower with ES (0% vs. 1.1%, p=0.0005).8 At 5 years, the rates of MACE were similar between the ES and SES groups (17.0% vs. 15.6%).9 The ENDEAVOR IV (Randomized Comparison of Zotarolimus-Eluting and Paclitaxel-Eluting Stents in Patients with Coronary Artery Disease) trial randomized patients with single coronary lesions to ES or paclitaxel-eluting stent (PES) groups.10 ES was non-inferior to PES for 9-month TVF: 6.6% and 7.1% for ES and PES, respectively. At the 5-year follow-up, however, the rate of death or MI was lower with ES (6.4% vs. 9.1%, p=0.048).11 In the ZEST (Comparison of the Efficacy and Safety of Zotarolimus-Eluting Stent with Sirolimus-Eluting and PacliTaxel-Eluting Stent for Coronary Lesions) trial patients with stable angina or non-ST elevation acute coronary syndrome were randomly assigned to the ES, SES, or PES groups.12 There was no significant difference in the rate of 12-month MACE between ES and SES (10.2% vs. 8.3%), but the 12-month MACE rate was lower in ES compared to PES (10.2% vs. 14.1%, p for superiority=0.01).
ER demonstrated good angiographic and clinical results in the First-In-Man trial. At the 9-month angiographic follow-up, ER had in-stent lumen late loss of 0.22±0.27 mm and in-stent angiographic binary restenosis of 1%. At 12 months, ER showed low clinical event rates: cardiac death of 0.7%, all MI of 5.8%, definite/probable stent thrombosis of 0%, TLR of 0.7%, TVF of 7.2%, and MACE of 8.7%.2
Even though the 2 versions of ZES showed good clinical outcomes, currently there is a paucity of clinical data directly comparing the ER and the ES. A retrospective study which conducted an indirect comparison of ES and ER suggested that ER, compared to ES, showed overall superior antirestenotic efficacy. However, both ES and ER were associated with a similar low risk of adverse safety events at 2 years.13 Similarly, in the present study, both ES and ER showed comparable results in overall MACE and stent thrombosis at 12 months. On subgroup analysis, the safety and efficacy of both ES and ER were consistent even in high risk patients undergoing multivessel PCI.
The present study has the usual limitations inherent in observational studies. Firstly, although these results come from a large cohort and adjustment was made using propensity score analysis including a large number of confounding variables, unmeasurable factors may still exist. Secondly, patients receiving ER had a higher prevalence of diabetes mellitus than patients treated with ES, indicating that treating physicians may have preferred the ER, the second ZES, for higher-risk patients with multivessel coronary artery disease. Thirdly, complete information was not available on some variables that may affect outcomes in our observational registry data including peripheral artery disease, chronic total occlusion, as well as the lower prevalence of bifurcation lesions and wide exclusion criteria such as patients with left main disease and lower left ventricular ejection fraction. Finally, the relatively small sample size may have underpowered the results of our study. In addition, shorter follow-up duration may have rendered it difficult to detect the clinical significance between the study groups.
In conclusion, in this large observational study with propensity-matched analysis, the safety and efficacy of both versions of ZES were comparable in patients with multi-vessel coronary artery disease during a 12-month clinical follow-up.
Figures and Tables
FIG. 1
Adjusted cumulative MACE at 12 months between ER and ES groups after propensity score matching by a Cox proportional hazards regression model stratified on matched pairs.
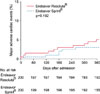
FIG. 2
Hazard ratios for 12-month MACE in propensity-matched cohort according to subgroup. CI: confidence interval, HR: hazard ratio.

TABLE 1
Baseline clinical characteristics between Endeavor Resolute® and Endeavor Sprint® groups before and after propensity score matching

TABLE 2
Characteristics of procedures and medical treatment during hospitalization between Endeavor Resolute® and Endeavor Sprint® groups before and after propensity score matching

Values are n (%), mean±SD or median (interquartile range). ACC/AHA: American College of Cardiology/American Heart Association, ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker, IVUS: intravascular ultrasound, PCI: percutaneous coronary intervention, TIMI: Thrombolysis in Myocardial Infarction.
References
1. Kandzari DE, Leon MB. Overview of pharmacology and clinical trials program with the zotarolimus-eluting endeavor stent. J Interv Cardiol. 2006; 19:405–413.

2. Meredith IT, Worthley S, Whitbourn R, Walters DL, McClean D, Horrigan M, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009; 2:977–985.

3. Williams PD, Awan M. Stent selection for percutaneous coronary intervention. Cont Cardiol Educ. 2017; 3:64–69.

4. Price MJ, Shlofmitz RA, Spriggs DJ, Haldis TA, Myers P, Popma Almonacid A, et al. Safety and efficacy of the next generation Resolute Onyx zotarolimus-eluting stent: primary outcome of the RESOLUTE ONYX core trial. Catheter Cardiovasc Interv. 2017; DOI: 10.1002/ccd.27322. [Epub ahead of print].

5. Kandzari DE, Leon MB, Popma JJ, Fitzgerald PJ, O'Shaughnessy C, Ball MW, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006; 48:2440–2447.

6. Eisenstein EL, Leon MB, Kandzari DE, Mauri L, Edwards R, Kong DF, et al. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009; 2:1199–1207.

7. Rasmussen K, Maeng M, Kaltoft A, Thayssen P, Kelbaek H, Tilsted HH, et al. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet. 2010; 375:1090–1099.

8. Maeng M, Tilsted HH, Jensen LO, Kaltoft A, Kelbæk H, Abildgaard U, et al. 3-Year clinical outcomes in the randomized SORT OUT III superiority trial comparing zotarolimus- and sirolimus-eluting coronary stents. JACC Cardiovasc Interv. 2012; 5:812–818.

9. Maeng M, Tilsted HH, Jensen LO, Krusell LR, Kaltoft A, Kelbæk H, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014; 383:2047–2056.

10. Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O'Shaughnessy C, et al. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010; 55:543–554.

11. Kirtane AJ, Leon MB, Ball MW, Bajwa HS, Sketch MH Jr, Coleman PS, et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013; 6:325–333.

12. Park DW, Kim YH, Yun SC, Kang SJ, Lee SW, Lee CW, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010; 56:1187–1195.
13. Tada T, Byrne RA, Cassese S, King L, Schulz S, Mehilli J, et al. Comparative efficacy of 2 zotarolimus-eluting stent generations: resolute versus endeavor stents in patients with coronary artery disease. Am Heart J. 2013; 165:80–86.

14. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356:1020–1029.

15. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17:2265–2281.
16. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999; 150:327–333.

17. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985; 39:33–38.

18. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161.

19. Cohen J. The t test for means. In : Cohen J, editor. Statistical poweranalysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates;1988. p. 19–74.
20. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001; 54:387–398.

21. Atalar E, Haznedaroğlu I, Aytemir K, Aksöyek S, Ovünç K, Oto A, et al. Effects of stent coating on platelets and endothelial cells after intracoronary stent implantation. Clin Cardiol. 2001; 24:159–164.

22. Malik N, Gunn J, Shepherd L, Crossman DC, Cumberland DC, Holt CM. Phosphorylcholine-coated stents in porcine coronary arteries: in vivo assessment of biocompatibility. J Invasive Cardiol. 2001; 13:193–201.
23. Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002; 23:1697–1706.

24. Udipi K, Melder RJ, Chen M, Cheng P, Hezi-Yamit A, Sullivan C, et al. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention. 2007; 3:137–139.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download