Abstract
Background
Incorrect administration of an anesthetic during local anesthesia is one of the most important causes of pain symptoms in patients scheduled for dental procedures. The current study assessed the severity of damage to periodontal tissue following different rates of anesthetic administration.
Methods
The research was conducted on 50 outbred male rats with a body mass of 180–240 g. The anesthetic used was 1% articaine.
Results
The results showed that administration of the anesthetic at a rapid pace caused structural damage to the periodontal tissue. Further, signs of impaired microcirculation were noted at all rates of administration. Biochemical studies demonstrated changes in the level of glucose and enzymes with the rapid introduction of the anesthetic, indicating severe systemic stress response of the body.
Dental or medical interventions in the maxillofacial area or the oral cavity, which can induce pain are an indication for local anesthesia. Administration of local anesthetics enables the dentist to perform the whole range of dental manipulations almost painlessly. However, the administration of an anesthetic itself is perceived as the most painful stage of treatment by most of the patients. Moreover, such sensations are often so pronounced that they cause delay or cancellation of the dental appointment [1234]. Administration of a local anesthetic without pain, discomfort, or apprehension can also prevent systemic complications such as elevated blood pressure or vasovagal syncope [5].
Currently, a whole range of supplemental methods intended to neutralize the painful effects of anesthetic administration, including application anesthetics, modified needles, and syringes, as well as different sedation methods are available [6]. However, while the efficiency of many of these methods (such as application gels, atraumatic needles) are not proven [7], the implementation of others (sedation) is not always economically or methodologically viable.
Pain while injecting an anesthetic can occur following: initial needle penetration into tissues [89], needle movement to the spot of anesthetic injection, and tissue swelling caused by the anesthetic injection [10]. Individual characteristics of the patient can also accentuate pain during the administration of local anesthesia [2]. Apart from these, pain symptoms during the administration of local anesthesia is reportedly determined by mechanical swelling of tissue following elevated intratissue pressure during the first few seconds of injection [11] and impairment of solution delivery [12]. Although the existing guidelines on anesthesia techniques recommend slow introduction of the anesthetic to minimize pain, it may not be always feasible in clinical practice [13141516].
A few studies have evaluated the rate of administration of anesthetic solution in clinical practice. It was reported that a doctor manages to smoothly administer anesthetic in only 14% of the cases, and the speed of administering an anesthetic depends on the gender (men administer anesthetic faster), experience (dentists with higher experience administer faster than students) and needle parameters (with 30 gauge needle, the time of anesthetic administration over 1 minute was noted in 75% instances, while it was noted only in 47.9% instances with a 27 gauge needle) [1718]. Skilled administration required for painless injection is not achieved in all manipulations. Irregularities such as failure to maintain straightness during introduction occur during the first three seconds of administering in most of the cases (75%), which is evident from the significant effort made by the dentist to move the plunger [11]. Additionally, the injection pressure while administering anesthesia fluctuates from 17,061 to 34,122 mm Hg, depending on the type of tissue and the individual experience [1920].
Therefore, it can be suggested that variation in anesthetic delivery is one of the most important causes of pain experienced by the patients during the administration of local anesthesia. However, there is no research assessing the acuteness of stress-induced reactions and oral mucosa damage in response to incorrect rate of anesthetic solution introduction.
Hence, the objective of the current study was to assess the severity of stress response and damage to periodontal tissue following different rates and techniques of administration (infiltration and intraligamentary) of a local anesthetic.
The study was carried out on healthy, sexually mature, nonlinear albino male rats. The animals used in the tests were quarantined in the vivarium of the Institute of Immunology and Physiology of the Ural Division of RAS (Yekaterinburg, Russia). The animals showed no symptoms of any disease. All animals were kept in standard conditions, and were fed according to customary schedule. All animals undergoing surgery received a similar level of care and attention. Aseptic technique and sterile instruments were used during the surgery.
All experimental procedures involving animals were approved by the Institute of Animal Care and Use Committee at the Institute of Immunology and Physiology of the Ural Division of RAS and were performed in accordance with the principles formulated in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, France, 18.03.1986), APS's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training, and the Laboratory Practice Regulations of RF (Ministry of Public Health Order no. 267 from 19.06.2003) [212223].
The study was conducted on 50 outbred male rats with a body mass of 180–240 g. The animals were categorized into 5 groups as described below, to obtain statistically valid results. Variation in terms of the initial mass among the groups did not exceed 10%.
Experimental group A (rapid introduction of anesthetic) consisted of 10 animals with an average mass of 223 ± 15.0 g. Experimental group B (slow introduction of anesthetic) consisted of 10 animals with an average mass of 256 ± 12.0 g. Experimental group C (fast intraligamentary introduction of anesthetic) consisted of 10 animals with an average mass 232 ± 11 g. Experimental group D (slow intraligamentary introduction of anesthetic during 1 minute) consisted of 10 animals with an average mass of 240 ± 10 g. The control group I consisted of 10 animals with an average mass of 220 g. The speed of injection was chosen in accordance with the well-known device, Anaeject® by Septodont®, which is widely used in clinics among the globe. The time of injection was about 30 sec in the group A, while it was about 1 minute in group B (slow mode of Anaeject®) [24].
The animals which were previously narcotized with 40 mg/kg aethaminalum-natrium, administered intraperitoneally, were taken off the experiment 1.5 hours after the initial manipulations.
The anesthetic solution used was 1% articaine (composition: ultracain D-S solution for injection in 2 ml ampule, solution for injection cartridge 1.7 ml: articaine hydrochloride―40 mg/ml; epinephrine hydrochloride―6 mkg/ml). Based on specific metabolism (accelerated compared to human) and mass of body of experimental animals (on average 240 g), 0.09–0.1 ml of the solution was considered as the administration volume.
The anesthetic was administered with a standard cartridge syringe using 12 mm long needle, in compliance with the recommendations on local anesthesia administration (cartridge single-use A (inch) 0.3 × 12 mm, DEPO JECT Korea).
About 3 ml of blood was taken for biochemical analysis by cardiocentesis with further centrifugation and serum separation. The samples were evaluated using a standard biochemical analyzer (BeckmanCoulter ImmunochemistrySystems, USA). Blood plasma was investigated for the following biochemical factors which serve as non-specific markers of stress reaction: glucose level and enzyme strength of serum glutamic oxalacetic transaminase or AST, and serum glutamic pyruvic transaminase or ALT. For biochemical analyses, ready-made reagents kits (Vital Diagnostics; SPb, Russia) were used. Histologic specimens of the periodontal tissue were prepared in accordance with standard practices, and colored with hematoxylin and eosin [2526].
The statistical software package, STATISTICA 6.0 (StatSoft, Inc. 2001) was used for data analysis. The data are presented in the form of arithmetic mean (M) ± standard error of mean (m). To test the hypothesis about homogeneity of two independent selections, the non-parametric Mann-Whitney U test was employed. When testing statistic hypotheses, 5% significance point was used.
A slight, yet a positive increase in the glucose level was noted in group A 1.5 hours after rapid introduction of articaine solution when compared to all other groups (Table 1), which differed from the glucose level in rats of group B (which underwent slow introduction of the medication) and the control group. The glucose indicator in animals of group C positively exceeded that of animals of the control group and group D. It is generally accepted that ALT and AST are the most reliable markers of liver injury, and they reflect injury and increase in membrane permeability of the hepatocytes. However, an increase in serum aminotransferase activity, apart from liver injury, can be caused by several other factors, including stress from strenuous exercise, dramatic weight loss, and hemolysis [27].
Verifiable changes of ALT activity in blood serum were not registered in any of the groups.
The animals of group A showed a positive increase of AST activity (almost 1.7 times higher) compared to intact rats following rapid introduction of the anesthetic, while the difference of this factor in group B from the control group was not verifiable. The animals of group C registered significantly higher increase in activity of this enzyme, compared to both group D and the control group.
No structural changes were noted in the gingival mucosa and intramucosal structures of the control group (Fig. 1-1).
Contrarily, surface disruption with exudative reaction, as evidenced by infiltration of the epithelium with segmental leukocytes was noted in group A samples (Fig. 1-2). In the deep tissues, focus of hemorrhage and disturbances in microcirculatory bloodstream vessels were registered in the form of focal adiemorrhysis and swelling and desquamation of endothelial cells (Fig. 1-3).
In experimental group B (with the slow introduction of the anesthetic), the structural changes in periodontal tissue were minimal, while the continuity of the epithelium was preserved. However, focal hyperemia was noted in the intramucosal microcirculatory vessels (Fig. 1-4).
Signs of intragingival ligament damage with moderate swollen adjacent gingival mucosa and submucosal edema were noted in group C (Fig. 2-1). No structural damage was registered in group D as compared to the control. However, moderate interstitial edema of connective tissues was registered in group D (Fig. 2-2).
In accordance with the results of the biochemical research, verified glucose escape was observed in animals from groups A and C (which underwent rapid introduction of the anesthetic into mucosa or intraligamentary tissues), as compared to the animals of control group I. However, this was not noted in animals of groups B and D. Seemingly, such glucose escape with the rapid introduction of the anesthetic suggested the development of stress reaction accompanied by energy resources mobilization at initial stages [29].
Changes in glucose level, apparently, cannot be explained by the increase in serum catecholamine levels [30], as it is dose-dependent and is manifested for several minutes to half an hour [31], while the biochemical analysis was conducted after 1.5 hours. It appears that it is a symptom of the progressing stress reaction, as also evidenced by changes in AST enzymes activity and increasing DeRitis coefficient [28]. It has been established that AST is a component of heart, liver, kidney, skeletal muscles, neural tissues, and that of pancreatic gland, spleen, and lungs, to a lesser degree. Hence, elevation of the level of this enzyme in the blood suggests progression of the initial stage of a generalized stress reaction [32].
According to the histological outcomes, signs of impaired microcirculation were registered at any speed of anesthetic introduction, which is an evidence of the progressing adaptation syndrome [33]. However, a possible cause of these disorders can be the presence of vasoconstrictor agents (epinephrine hydrochloride) used widely in the local anesthetics.
Vasoconstrictor agents are commonly integrated in anesthetic solutions to facilitate vasospasm at the spot of injection, which in turn prevents rapid reduction of the anesthetic, thereby potentiating its function. Additionally, these agents result in prolongation and enhancement of analgesic effect due to the inhibitory action on the myelinated fibres [3435]. Use of vasoconstrictive components also enables reduction of the overall concentration of the anesthetic in the body and prevents progression of central toxic reactions [3435].
Nevertheless, morphological changes are noted at the spot of injection, regardless of the amount of the introduced medication (with vasoconstrictor), suggesting the progression of aseptic coagulation necrosis along with progressive reactive inflammation in the area of medication introduction [36].
However, as noted in the current study, progression of necrosis was not obligatory and was dependent on the speed of introduction of the anesthetic. At a slow speed of anesthetic solution introduction, minimal signs of response reaction in the form of capillary vessel constrictions and formations of erythrocyte “sludge complexes” (aggregation and agglutination of RBCs) were registered (group B, Fig. 1-4).
Such changes in the microcirculatory bloodstream were also registered by other researchers, who suggested that with an adrenaline concentration of 1:50,000, complete adiemorrhysis occurs at the spot of introduction, which progresses for 20–30 s and continues for 15–20 min [3738]. Nevertheless, surface epithelium disruption with developing exudative reaction was observed only with the rapid introduction of anesthetic in the current study.
Presence or absence of necrosis symptoms following the introduction of the anesthetic, apart from the speed of infusion, also depends on the type of subiculum and the pressure of solution injection [3738]. Hence, the appearance of the necrotic changes at the spots of introduction of anesthetics with vasoconstrictors as described in literature [36] can be explained by the inadequacy of the model used by the authors (anesthetic is injected into muscular tissue and not into mucosa or dentogingival ligament).
Similarly, when administering intraligamentary anesthesia, regardless of the speed of the introduction of the anesthetic (groups C and D, Fig. 1 and 2), destruction of the dentogingival ligament was observed, which could be attributed to the tear of tissues due to high pressure of the solution noted while administering this type of anesthesia.
In conclusion, injection of local anesthetic at any speed of introduction induces response in the form of vascular congestion in the microcirculatory bloodstream and exudative reactions.
Rapid introduction of an anesthetic causes progression of structural disorders of gingival tissue.
Changes in the levels of glucose and enzymes following rapid introduction of local anesthetics signify the severe systemic stress reaction of the body with this type of introduction.
ACKNOWLEDGMENTS
The study was supported by the Government contract Russian Federation with Institute of Immunology and Physiology (AAAA-A18-118020690020-1), and, partly, by the Act 211 of the Government of Russian Federation, contract No 02.A03.21.0006.
References
1. Milgrom P, Coldwell SE, Getz T, Weinstein P, Ramsay DS. Four dimensions of fear of dental injections. J Am Dent Assoc. 1997; 128:756–762. PMID: 9188235.

2. Abrahamsson KH, Berggren U, Hallberg L, Carlsson SG. Dental phobic patients' view of dental anxiety and experiences in dental care: a qualitative study. Scand J Caring Sci. 2002; 16:188–196. PMID: 12000673.

3. Weinstein P, Shimono T, Domoto P, Wohlers K, Matsumura S, Ohmura M, et al. Dental fear in Japan: Okayama Prefecture school study of adolescents and adults. Anesth Prog. 1992; 39:215–220. PMID: 8250343.
4. Tzafalia M, Sixou JL. Administration of anesthetic solutions using a metal syringes. An ex vivo study. Anesth Prog. 2011; 58:61–65. PMID: 21679041.
5. Saijo M, Ito E, Ichinohe T, Kaneko Y. Lack of pain reduction by a vibrating local anesthetic attachment: a pilot study. Anesth Prog. 2005; 52:62–64. PMID: 16048153.

6. Jacobs S, Haas DA, Meechan JG, May S. Injection pain: comparison of the three mandibular block techniques and modulation by nitrous oxide. J Am Dent Assoc. 2003; 134:869–876. PMID: 12892444.
7. Flanagan T, Wahl MJ, Schmitt MM, Wahl JA. Size doesn't matter: needle gauge and injection pain. Gen Dent. 2007; 55:216–217. PMID: 17511363.
8. Meechan JG, Howlett PC, Smith BD. Factors influencing the discomfort of intraoral needle penetration. Anesth Prog. 2005; 52:91–94. PMID: 16252738.

9. Nakanishi O, Haas D, Ishikawa T, Kameyama S, Nishi M. Efficacy of mandibular topical anesthesia varies with the site of administration. Anesth Prog. 1996; 43:14–19. PMID: 10323120.
10. Kaufman E, Epstein JB, Naveh E, Gorsky M, Gross A, Cohen G. A survey of pain, pressure, and discomfort induced by commonly used oral local anesthesia injections. Anesth Prog. 2005; 52:122–127. PMID: 16596910.

11. Meechan JG. Pain control in local analgesia. Eur Arch Paediatr Dent. 2009; 10:71–76. PMID: 19627670.

12. Primosch RE, Brooks R. Influence of anesthetic flow rate delivered by the Wand Local Anesthetic System on pain response to palatal injections. Am J Dent. 2002; 15:15–20. PMID: 12074223.
13. Kudo M. Initial injection pressure for dental local anesthesia: effects on pain and anxiety. Anesth Prog. 2005; 52:95–101. PMID: 16252739.

14. Pashley EL, Nelson R, Pashley DH. Pressures created by dental injections. J Dent Res. 1981; 60:1742–1748. PMID: 6944338.

15. Sumer M, Misir F, Koyuturk AE. Comparison of the Wand with a conventional technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e106–e109. PMID: 16731373.

16. Versloot J, Veerkamp JS, Hoogstraten J. Computerized anesthesia delivery system vs. traditional syringe: comparing pain and pain-related behavior in children. Eur J Oral Sci. 2005; 113:488–493. PMID: 16324138.

17. Meechan JG. Differences between men and women regarding attitudes toward dental local anesthesia among junior students at a United Kingdom dental school. Anesth Prog. 2005; 52:50–55. PMID: 16048151.

18. Rankin JA, Harris MB. Comparison of stress and coping in male and female dentists. J Dent Pract Adm. 1990; 7:166–172. PMID: 2084220.
19. Hochman MN, Friedman MJ, Williams W, Hochman CB. Interstitial tissue pressure associated with dental injections: a clinical study. Quintessence Int. 2006; 37:469–476. PMID: 16752703.
20. Walmsley AD, Lloyd JM, Harrington E. Pressures produced in vitro during intraligamentary anaesthesia. Br Dent J. 1989; 167:341–344. PMID: 2590570.

21. Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. The 1996 Guide for the care and use of laboratory animals. ILAR J. 1997; 38:41–48. PMID: 11528046.

22. Flecknell PA. Laboratory animal anesthesia. London: Academic Press;1987.
23. Swindle MM, Adams RJ. Experimental surgery and physiology: induced animal models of human disease. Baltimore: Williams & Wilkins;1988.
24. Baart JA, Brand HS. Microprocessor-Aided Local Anaesthesia. Local Anaesthesia in Dentistry. 2Ed. Springer International Publishing;2017. p. 113–123.
25. Lillie RD. HJ Conn's Biological Stains. 4th ed. Baltimore: Williams and Wilkins;1977.
26. Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. 4th ed. New York: Churchill Livingstone;1996.
27. Pratt DS, Kaplan MM. Evaluation of abnormal liver enzyme results in asymptomatic patients. N Engl J Med. 2000; 342:1266–1271. PMID: 10781624.
28. De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. 1957. Clin Chim Acta. 2006; 369:148–152. PMID: 16781697.
29. Selye H. Stress in health and disease. Boston: Butterworths;1976.
30. Cioffi GA, Chernow B, Glahn RP, Terezhalmy GT, Lake CR. The hemodynamic and plasma catecholamine responses to routine restorative dental care. J Am Dent Assoc. 1985; 111:67–70. PMID: 3861687.

31. Jastak JT, Yagiela JA. Vasoconstrictors and local anesthesia: a review and rationale for use. J Am Dent Assoc. 1983; 107:623–630. PMID: 6355236.

32. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002; 123:1364–1366. PMID: 12360497.
34. Grizuk SF. Oral and Maxillofacial Surgery. Moscow: Geotar-Media;2010. [In Russian].
35. Sisk AL. Vasoconstrictors in local anesthesia for dentistry. Anesth Prog. 1992; 39:187–193. PMID: 8250339.
36. Ibragimov ZI, Semkin VA, Didikin SS, Titova GP. Features of the morphological changes of skeletal muscles after intramuscular administration of anesthetics. Alfavit stomatologii. 2007; 1:42–44. [In Russian].
37. Zoryan EV, Rabinovich SA. Vasoconstrictive agents in composition of local anesthetics: significance and problems. Clin Dent. 2006; 3:24–28. [In Russian].
38. Rabinovich SA, Kuznetzov GI, Moscowez ON, Zoryan EV. Int Dent Rev. 2008; 1:14–15. [In Russian].
Fig. 1
Periodontal tissues. Hematoxylin and eosin (H&E) staining (200 ×). (1-1) Control. Histological changes are absent, (1-2) Rapid introduction of the drug. Severe infiltration (arrow) and signs of aseptic inflammation, (1-3) Rapid introduction of the drug. Severe infiltration and signs of aseptic inflammation, microvascular spasm. The splitting of the epithelium, (1-4) Slow injection of the drug. Minimal signs of reaction from the bloodstream in the form of spasm of the capillaries (arrow) and the formation of “sludge complexes” (aggregation and agglutination of red blood cells).
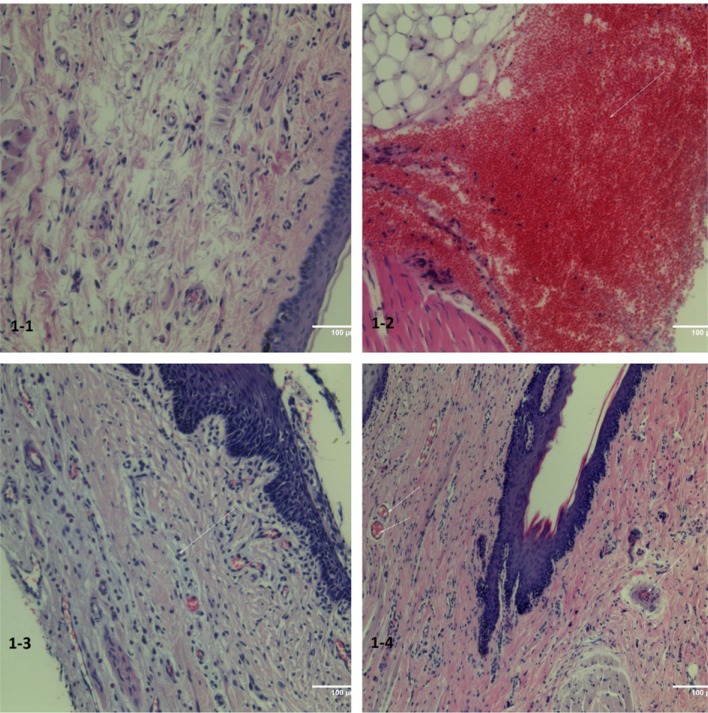
Fig. 2
Fast intraligamentary introduction of the anesthetic solution. (2-1) Destruction of the dentogingival ligament (A) with moderate edema of the mucosa and submucosa (B) of the adjacent gums. (2-2) Destruction of the dentogingival ligament (B) with moderate edema of the mucosa and submucosa (A) of the adjacent gums. Hematoxylin and eosin (H&E) staining (200 ×).
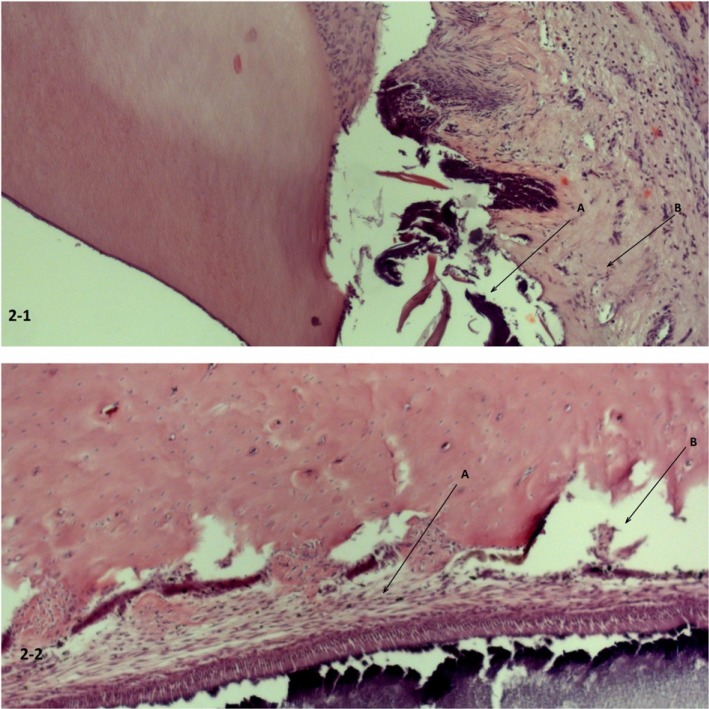
Table 1
Results of biochemical tests (Mean ± SEM)
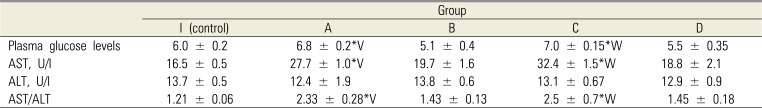
Statistically significant difference in values in case of paired comparison: *P < 0.05 in comparison with control animals; V – P < 0.05 in comparison between groups A and B; W – P < 0.05 in comparison between groups C and D. U/l—units per liter. AST, aspartate aminotransferase; ALT, alanine aminotransferase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download