1. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet. 2008; 372:139–144. PMID:
18582931.

2. Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, Hert SD, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: Cardiovascular assessment and management: The joint task force on non-cardiac surgery: Cardiovascular assessment and management of the european society of cardiology (ESC) and the european society of anaesthesiology (ESA). Eur Heart J. 2014; 35:2383–2431. PMID:
25086026.
3. Irita K, Kawashima Y, Seo N, Morita K, Iwao Y, Tsuzaki K. Cardiac arrest in the operating theatre and its outcome: An analysis of 3,855,384 anesthetics over 4 years in japanese society of anesthesiologists - certified training hospitals: A-28. Eur J Anaesthesiol. 2004; 21:8.
4. Biboulet P, Aubas P, Dubourdieu J, Rubenovitch J, Capdevila X, d'Athis F. Fatal and nonfatal cardiac arrests related to anesthesia. Can J Anaesth. 2001; 48:326–332. PMID:
11339772.
5. Olsson GL, Hallen B. Cardiac arrest during anaesthesia. A computer-aided study in 250,543 anaesthetics. Acta Anaesthesiol Scand. 1988; 32:653–664. PMID:
3213390.
6. Newland MC, Ellis SJ, Lydiatt CA, Peters KR, Tinker JH, Romberger DJ, et al. Anesthetic-related cardiac arrest and its mortality: A report covering 72,959 anesthetics over 10 years from a us teaching hospital. Anesthesiology. 2002; 97:108–115. PMID:
12131111.
7. Rukewe A, Fatiregun A, Osunlaja TO. Cardiac arrest during anesthesia at a university hospital in nigeria. Niger J Clin Pract. 2014; 17:28–31. PMID:
24326803.

8. You TM, Kim S. Pulseless electrical activity during general anesthesia induction in patients with amyotrophic lateral sclerosis. J Dent Anesth Pain Med. 2017; 17:235–240. PMID:
29090256.

9. Messahel FM, Al-Qahtani AS. Incidence of perioperative cardiac arrest: Analysis of anesthetics over 18-year period. Middle East J Anaesthesiol. 2010; 20:815–819. PMID:
21526666.
10. Oberman A, Kouchoukos NT, Holt JH Jr, Russell RO Jr. Long-term results of the medical treatment of coronary artery disease. Angiology. 1977; 28:160–168. PMID:
869279.

11. Chiang S, Cohen B, Blackwell K. Myocardial infarction after microvascular head and neck reconstruction. Laryngoscope. 2002; 112:1849–1852. PMID:
12368628.

12. Pratt GF, Faris JG, Lethbridge M, Teh LG. Breast reconstruction with a free diep (tram) flap complicated by cardiac tamponade and arrest: A case report. J Plast Reconstr Aesthet Surg. 2009; 62:e73–e75. PMID:
19081309.

13. Young L, Myers LL. Free flap reconstruction of oral composite defect complicated by intraoperative cardiac arrest: A case report. Microsurgery. 2013; 33:236–238. PMID:
23255281.

14. Bicsak A, Jellen A, Tuppy H, Poeschl WP. Sudden death after major head and neck cancer surgery due to undetected arrhythmogenic right ventricle cardiomyopathy (ARVC). Oral Maxillofac Surg. 2016; 20:431–434. PMID:
27357590.

15. Ha W, Kim KN, Ann SH, Yoon CS. Sudden cardiac death with myocardial infarction after free-flap lower extremity reconstruction. Chin Med J (Engl). 2017; 130:499–500. PMID:
28218229.

16. Celik T, Yuksel UC, Kursaklioglu H, Iyisoy A, Kose S, Isik E. Precordial st-segment elevation in acute occlusion of the proximal right coronary artery. J Electrocardiol. 2006; 39:301–304. PMID:
16777516.

17. Acikel M, Yilmaz M, Bozkurt E, Gurlertop Y, Kose N. St segment elevation in leads v1 to v3 due to isolated right ventricular branch occlusion during primary right coronary angioplasty. Catheter Cardiovasc Interv. 2003; 60:32–35. PMID:
12929099.
18. Adesanya AO, de Lemos JA, Greilich NB, Whitten CW. Management of perioperative myocardial infarction in noncardiac surgical patients. Chest. 2006; 130:584–596. PMID:
16899865.

19. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999; 100:1043–1049. PMID:
10477528.

20. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015; 373:2258–2269. PMID:
26630144.

21. Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: A quantitative systematic review. Anesth Analg. 2003; 97:623–633. PMID:
12933373.

22. Martinez EA, Kim LJ, Faraday N, Rosenfeld B, Bass EB, Perler BA, et al. Sensitivity of routine intensive care unit surveillance for detecting myocardial ischemia. Crit Care Med. 2003; 31:2302–2308. PMID:
14501960.

23. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007; 50:2173–2195. PMID:
18036459.
24. Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult advanced cardiovascular life support: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132:S444–S464. PMID:
26472995.
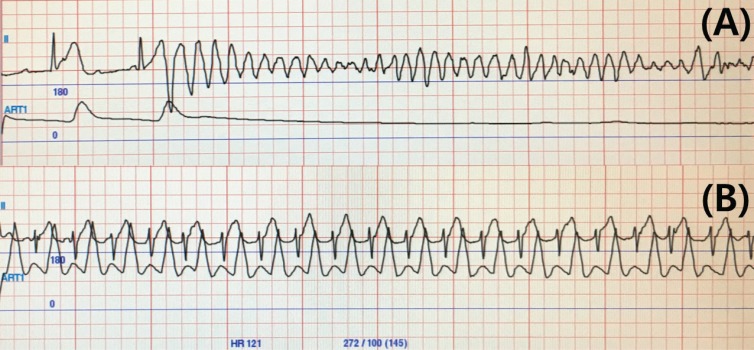
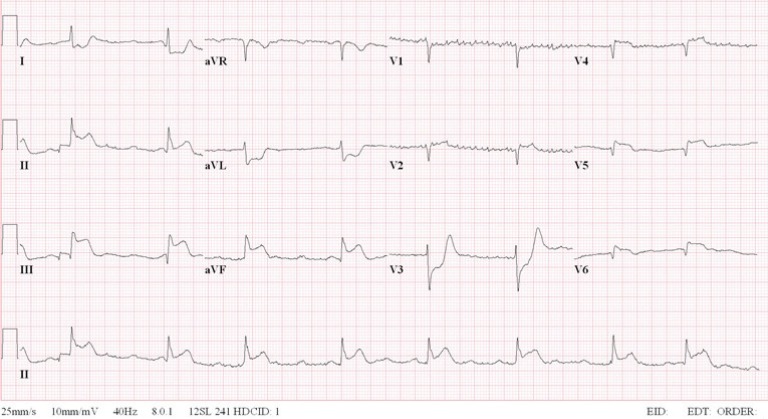
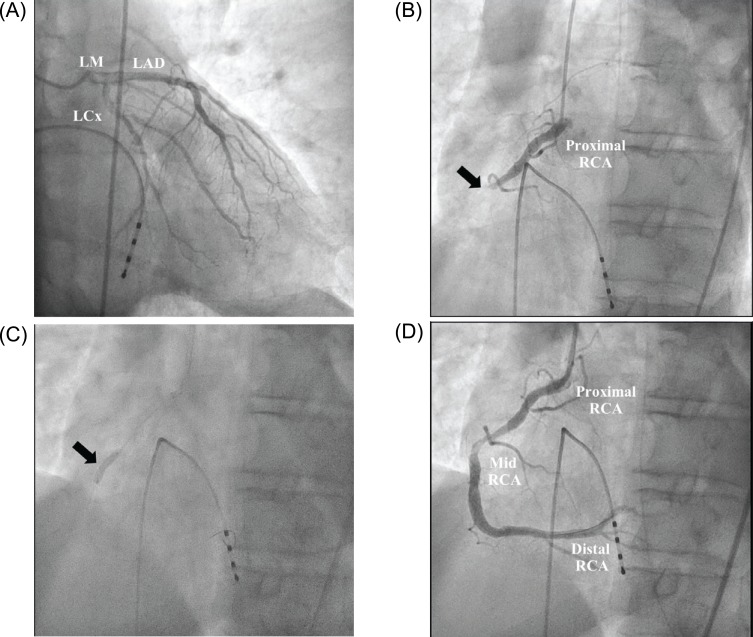
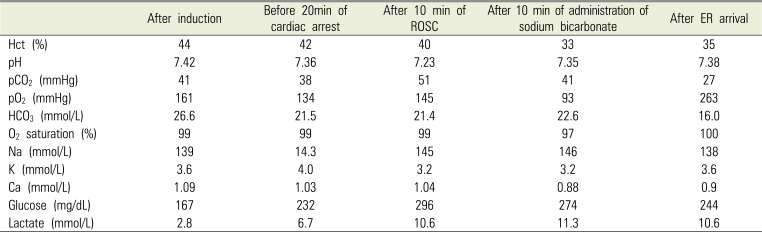




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download