Abstract
Noise in the knee joint is a common symptom that often leads to outpatient clinic visits. However, there have been no previous review articles regarding noise around the knee despite its high prevalence. We will review the noise characteristics according to sound nature and onset as well as factors for differentiation between physiological and pathological noises. In addition, we will describe causes of the physiological and pathological noises and management of noise in the knee. An appropriate review of the characteristics of noise, its pathophysiology, and factors for differentiation between physiological and pathological noises can facilitate patient guidance. It is important to differentiate between physiological noise and pathologic noise. In most cases, noise after surgery is simply the perception of noise that had been present previously due to emotional concerns. Minor problems associated with surgery, such as postoperative noise, can decrease patient satisfaction, especially among patients with high expectations. Following surgical principles and providing accurate information about physiological noise can decrease the risk of both pathological noise and patient dissatisfaction. In total knee arthroplasty, every attempt should be made to avoid patellar crepitus and clunk by using modern prostheses with proper patellofemoral conformity and by avoiding surgical errors.
Noise around the knee is common when performing repetitive knee extension and flexion or squats on the floor. A lot of patients visit outpatient clinics due to a concern that the noise is pathological. In most cases, the noise is physiological, and it is sufficient to explain the cause of the noise to patients and reassure them. However, clinicians should be aware that noise can be one of the symptoms of pathological lesions. Crema et al.1) reported that several structural pathologies of the knee were associated with an increased risk of general crepitus in osteoarthritis (OA). Schiphof et al.2) also suggested that crepitus may represent the first symptom of patellofemoral OA. Pathological noise requires appropriate treatment for baseline lesions. Therefore, it is important to differentiate between physiological noise and pathological noise, and to perform diagnostic interventions only in selected patients.
There have been few systematic reports of the prevalence of the noise in the knee. In a study involving people over 40 years of age, crepitus was reported in 38.1% of women and 17.1% of men.3) Unfortunately, the prevalence was limited to the specific population, not to the entire population in that age group. The prevalence of the noise in certain situations, especially in pathological conditions, has been more frequently reported. Bae et al.4) reported that painful popping occurred in 96.5% of patients diagnosed with posterior root tears of the medial meniscus. Patellar crepitus has been reported to occur in up to 18% of patients with posterior-stabilized total knee arthroplasty (TKA).56) Considering these previous studies, physiological noise and even pathological noise around the knee seem to be frequent symptoms.
To our knowledge, there have been no previous review articles regarding noise around the knee despite its significance and high prevalence. Hence, we review the noise characteristics according to sound nature and onset, as well as factors for differentiation between physiological and pathological noises. In addition, we describe causes of the physiological noise and pathological noise and management of the noise in the knee.
The sounds around the knee have been described using various terms, including popping, snapping, catching, clicking, crunching, cracking, crackling, creaking, grinding, grating, and clunking. These terms can be used to differentiate noises in terms of frequency, duration, and loudness, but it is not easy to describe the nature of the sounds precisely. The term “popping” is used to describe a sudden sharp explosion and well perceived sound in an injury situation; popping can occur at the root tear of the degenerative medial meniscus and at the detachment of the cruciate or collateral ligaments of the knee.7) The term “clunking” is used to describe a loud, singular noise occurring due to release against resistance; patellofemoral clunking after TKA is caused by entrapment of fibrotic nodule at the junction of the superior pole of the patella and the distal quadriceps tendon within the superior aspect of the intercondylar box of the femoral component during knee flexion to extension.8) The term “clicking” is used to describe a tiny, singular noise that occurs during one cycle of knee extension and flexion; it is commonly associated with meniscal tears.1) The terms “grinding” and “grating” are used to describe a continuous scratching noise; grinding or grating is common in degenerative OA and patellofemoral pain syndrome.29) However, joint sounds are commonly labeled as crepitus by doctors because this term is familiar due to the use for describing lung sounds.
The precise onset of physiological noise is commonly unknown by patients. On the contrary, the onset of pathological noise is relatively clear compared to physiological noise. The onset of pathological noise can be divided into acute and chronic. Acute onset of noise accompanied by pain may be caused by meniscal or ligament injury.710) Chronic pathological noise can occur gradually; it may occur sporadically or frequently, depending on the cause. Recurrent and chronic noise may be caused by old meniscal tears, cartilage injury, OA, patellofemoral instability, or patellofemoral pain syndrome.6111213)
The best way to differentiate between physiological noise and pathological noise is by checking for pain and swelling/effusion in the knee joint.11415) In addition, physiological noise has no association with a history of injury, no aggravation of sounds and combined symptoms, and a sporadic nature due to buildup of air in the joint fluid.1617)
However, pathological noise is often associated with pain and swelling/effusion in various intra- and extra-articular pathologies. A loud “pop” with pain at the time of injury usually indicates damage to the ligaments or the meniscus.7) Crepitus, in the absence of any history of injury, may indicate cartilage lesions in OA or inflammatory arthritis.218) In addition, pathological noise can be observed consistently on careful examination, instead of being intermittent in nature like air-related physiological noise.
A great deal of research has been done to detect noise around the knee, and to compare physiological and pathological noise by acoustic emission.192021) Friction between articulating structures, as occurs at the knee joint, gives rise to various types of vibrations. This acoustic energy travels to the surface of the skin, encountering a large impedance mismatch between the fluid-filled tissue and air.192021) Although the majority of the acoustic energy is reflected back into the tissue, a small amount propagates into the air, resulting in audible sounds. Vibration sensors (e.g., accelerometers, piezoelectric devices, and stethoscopes) or air microphones (e.g., electret microphones and microelectromechanical systems) are used to measure joint vibration sounds.21)
Numerous previous studies developed diagnostic techniques by acoustic emission for differentiation between physiological and pathological noises.192021) It was reported that OA knees produce acoustic emissions with greater frequency, higher peaks, and longer duration compared to healthy knees.192021) Shark et al.20) compared the acoustic emissions between age-matched healthy and OA knees during the four phases of the sit-stand-sit movement, and found significant differences in the peak magnitude and average signal level of each acoustic emission burst, especially during the ascending deceleration phase. Prior et al.19) compared the frequency of joint acoustic events between clinically healthy and OA knees, where joint acoustic events were defined as burst signal waveforms above a threshold of 32 dB. They reported that clinically healthy knees could be distinguished from OA knees according to the number of joint acoustic events and their frequencies.
However, the method of using acoustic emission needs much improvement to be more useful in clinical practice. Future works should be performed to mitigate background and interface noise, and develop new processing techniques for detecting clinically relevant acoustic signatures.22) Further studies will be required to determine the specific acoustic emission features that provide valuable information.
The origin of physiological noise varies and includes a buildup or bursting of tiny bubbles in the synovial fluid, snapping of ligaments, catching of the synovium or physiological plica, hypermobile meniscus or discoid meniscus, and perception of previous noise after knee surgery due to emotional concerns (Table 1).1013152324252627)
Joint sounds occurring repeatedly with range of motion typically arise when anatomical structures rub against each other, but cracking sounds have a refractory period before being repeated, even with ongoing motion.2728) Although various hypotheses have been proposed regarding the origin of cracking sounds, the underlying mechanism remains unknown. Several authors suggested that the sudden collapse of a cavitation bubble caused cracking,172930) while others reported that the formation of a clear space or bubble was the source of cracking.28313233) Recently, Kawchuk et al.27) presented direct evidence from real-time magnetic resonance imaging (MRI) that the mechanism of cracking is related to cavity formation rather than bubble collapse. This is consistent with tribonucleation, a process where opposing surfaces resist separation until a critical point, at which they separate rapidly creating a sustained gas cavity.2731) Changes in joint pressure can cause tiny bubbles of gas to slowly form in the joints. When these gas bubbles are formed quickly, they make a popping sound. This type of air-mediated popping can also occur in joints other than the knee, and is more prevalent in the knuckle joints of the hands.29) This type of noise, caused by air buildup in the joint, is sporadic in nature.
Ligaments and tendons around the knee joint may stretch slightly as they pass over a small bony lump and then snap back into place, causing a clicking sound in the knee. Snapping syndrome of the knee joint is generally caused by the biceps femoris tendon at the lateral aspect of the normal knee joint.
Other factors can also create physiological noise. Demirag et al.25) reported that the physiological popping or snapping was present in 72% of patients with infrapatellar plica. A young child under 10 years of age with a hypermobile or discoid meniscus has intermittent experiences of popping and snapping without obvious pain.10) A previously present physiological noise may come to a patient's attention after minor trauma or knee surgery due to increased awareness based on heightened concern. Such noises include cracking, catching, and snapping. Reassurance, prevention of arthrofibrosis, and promotion of rehabilitation are sufficient to manage these conditions.
The causes of pathological noise include degenerative changes, pathological plica, patellofemoral instability, pathological snapping knee syndrome, and postsurgical crepitus (Table 1).125)
The OA knee shows gradual loss of cartilage, accompanying the development of bony spurs and cysts at the margins of the joints; in combination, all these structural pathologies may cause crepitus. Previous studies have investigated which pathologies, in various structures of the knee joint, are related to crepitus. With a model for the diagnosis of early knee OA, Crema et al.1) recruited 255 subjects with knee pain and compared structural pathology on MRI between groups with and without crepitus (180 and 75 knees, respectively) using multiple logistic regression analysis. An increased risk of compartment-specific crepitus was associated with osteophytes at medial and lateral tibiofemoral joints and the patellofemoral joint. Crepitus was also associated with medial collateral ligament pathology at the medial tibiofemoral compartment, but it was negatively associated with cartilage damage at the medial tibiofemoral compartment. In their whole-knee model, only meniscal tears were associated with an increased risk of general crepitus. Schiphof et al.2) analyzed the relationships between specific clinical findings and MRI-detected features of the patellofemoral OA joint. Clinical findings included pain at the patellar edges, quadriceps tendon and patellar tendon, compression test, crepitus, pain history, and current pain; crepitus in the knee was significantly associated with all of the prevalent MRI features of the patellofemoral OA joint, i.e., cartilage lesions, osteophytes, cysts, and bone marrow lesions. The authors suggested that crepitus is the first symptom of patellofemoral OA.
The plica is a fold in the synovium that represents an embryological remnant of the development of the synovial cavity in the knee.15) Although the reported prevalence of medial plica ranges widely from 22% to 95%,3435) the pathological plica, characterized by inflammation, thickening, and/or reduced elasticity, can produce clinically relevant pain and crepitus. Friction between the medial plica and the facing medial femoral condyle, occurring during activities of daily living, can increase the severity of pathological changes in the medial plica and give rise to clinical symptoms, such as synovitis and pain.35) Therefore, crepitus in the medial plica area requires attention if it produces a painful click.153435)
Patellofemoral instability is a widely defined clinical entity with a multifactorial etiology, including valgus or rotational deformity of the lower extremity, structural anomaly of the patella or femoral trochlea, soft tissue defects of the medial patellofemoral ligament, dysplasia of the vastus medialis obliquus, hypertrophy of the vastus lateralis, and tightness of the lateral retinaculum.36) Patellofemoral instability is associated with noise, due to hypermobility of patella or a reduction in the previously subluxated patella during active or passive flexion and extension of the knee. Smith et al.13) assessed the accuracy of physical examinations for diagnosing patellofemoral instability and showed very poor inter- and intraobserver agreement in the majority of the physical tests, with fair to moderate agreement seen only for assessments of patellofemoral crepitus. With regard to interobserver reliability, the assessment of crepitus was better physical examination than pain on palpation of the patellar retinaculum, the patellar compression test, Bassett's sign, and the vastus medialis obliquus capability test. Furthermore, it showed better intraobserver reliability than the patellar tracking test (J-sign).
It is necessary to determine the cause of snapping sounds around the knee joint in patients in which the sound is accompanied by pain. Various extra- and intra-articular structures can cause pathological snapping sounds in the knees. The pathologies of the intra-articular structures include ganglion cyst, lipoma, and synovial nodule.101537) The pathologies of the extra-articular structures include fabella, osteophytes, osteochondromas, and tendinopathy of the popliteus, biceps femoris, or pes anserinus tendons.23263839)
Various surgical procedures of the knee can cause the postsurgical noise. The crepitus can be presented when chondrocalcinosis occurs in postmeniscectomy knees.40) Singh et al.41) described that inflammation and impingement of Hoffa's fat pad after arthroscopy could cause crepitus. Paulos et al.42) reported the crepitus in infrapatellar contracture syndrome which most commonly occurred after reconstruction of the anterior cruciate ligament but might arise after less complex arthroscopic procedures including meniscectomy, lateral retinacular release, and diagnostic arthroscopy.
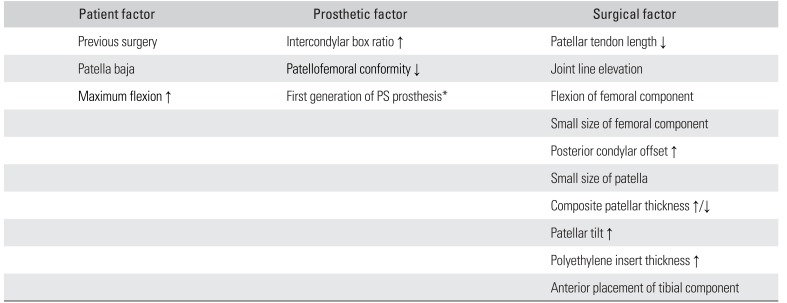
However, patellofemoral crepitus or clunk after TKA will be one of the most important issues after orthopedic surgeries. Various factors related to the patient, prosthesis, and surgical technique can influence patellofemoral crepitus/clunk after TKA (Table 2).5) With regard to patient factors, a previous surgery before TKA, patella baja, and an increased postoperative flexion angle may increase the risk of patellofemoral crepitus/clunk. As prosthetic factors, the increased intercondylar box ratio of the femoral component, reduced patellofemoral conformity, and a first-generation posterior-stabilized femoral component may also increase the risk of patellofemoral crepitus/clunk. Selection of contemporary femoral components with patellofemoral conformity has been shown to decrease the risk of patellar crepitus/clunk and patellofemoral instability.5) Several advantages associated with modification of the patellofemoral design, such as a decreased incidence of anterior knee pain and patellar crepitus, have been demonstrated in clinical studies.434445)
Factors related to the surgical technique include changes in patellar tendon length, joint line elevation, flexion of the femoral component, a small-sized femoral component and patella, decreased or increased thickness of the patellar component composite, increased patellar tilt and translation, and anterior displacement of the tibial component.56) These are clinically important because surgeons can address the risk of patellofemoral crepitus/clunk only through surgical technique-related factors. Nam et al.46) reported that patients frequently perceive noise emanating from the knee after TKA, while those reporting noise from the knee were more likely to have functional limitations and exhibit a limp, swelling, and stiffness. The authors suggested that surgeons should preoperatively inform patients of this possibility because unmet patient expectations have a negative impact on patient satisfaction after TKA.
Taking these factors associated with patellofemoral crepitus/clunk and possible functional limitation into consideration, it is important to avoid surgical errors and to use modern prostheses with extended trochlear groove geometry and proper patellofemoral conformity.
Again, it is important to differentiate between physiological and pathological noises. Physiological noise in the knee is common, but usually painless and harmless. If there is no pathological condition, there is no need to be concerned about the noise. It is sufficient to explain the cause of the noise to patients and reassure them. Various exercises for stretching and strengthening the musculotendinous structures may be beneficial for especially ligament snapping. Hip flexor, iliotibial band, and calf muscle stretching are common stretching exercises, while side steps performed with a resistance band, inner thigh squats, and vastus medialis obliquus activation are popular strengthening exercises.2426)
The management of pathological noise will depend on the underlying cause. It requires the conservative or surgical treatment to care for the cause of noise. OA has a variety of treatment options including reducing inflammation, appropriate protection of further damage to pathologic structures, physical therapy and strengthening of the muscles that support the knee.47) The pathological plica can be excised by arthroscopy if it makes refractory and persistent pain in spite of nonsurgical treatment.25) Many surgical procedures for the patellar instability have been introduced, but the surgical method should be reserved until conservative treatment has failed and the chronic recurrent nature of the disease has caused significant functional improvement. Surgical procedure may address either bony or soft tissue components, in a proximal or distal procedure; lateral release, medial repair, medial patellofemoral ligament reconstruction, tibial tubercle realignment, trochleoplasty can be performed.48) The snapping knee symptoms caused by an intra-articular ganglion cyst can be treated by mass excision.37) Surgical tenotomy or stabilization can be considered in snapping caused by semitendinosus, gracilis, and biceps femoris tendon.4950)
The postoperative pathological noise with symptoms can lead to dissatisfaction and functional limitation, especially among patients with high expectation. The possibility of the noise should be informed to the patients. Following surgical principles and providing accurate information can decrease the risk of pathological noise and dissatisfaction. The postoperative Hoffa's disease with pathological noise can be mostly managed by conservative treatment.41) Chronic stage with persistent symptoms or failure with conservative management may require arthroscopic or open resection of the hypertrophic fat pad.51) Severe infrapatellar contracture syndrome after arthroscopic procedures may require opening intra- and extra-articular debridement and release.42) Painful patellar crepitus/clunk after TKA can be effectively treated with either an open or arthroscopic debridement.6)
Noise around the knee is a common phenomenon. In most cases, the sound is physiological, and there is generally no reason for concern. Pathological noise is accompanied by pain, effusion, swelling, and a history of injury. It is characterized by high frequency and gradual aggravation. It is important to differentiate pathological noise from physiological noise. Healthy patients experiencing physiological noise should be given appropriate information and reassurance, while diagnostic interventions for pathological noise should be applied in selected patients. Careful evaluation of the characteristics of noise and differentiation between physiological and pathological noises can provide guidance for patients. Most noises after surgery involve perception of noise that had already been present due to emotional concerns. Minor problems associated with surgery, such as postoperative noise, can reduce patient satisfaction, especially in patients with high expectations. Following surgical principles and providing appropriate information about physiological noise can reduce the risk of pathological noise and unnecessary anxiety, resulting in good patient satisfaction. In TKA, patellar crepitus and clunk should be avoided by using modern prostheses with good patellofemoral conformity and by avoiding surgical errors.
References
1. Crema MD, Guermazi A, Sayre EC, et al. The association of magnetic resonance imaging (MRI)-detected structural pathology of the knee with crepitus in a population-based cohort with knee pain: the MoDEKO study. Osteoarthritis Cartilage. 2011; 19(12):1429–1432. PMID: 21945851.

2. Schiphof D, van Middelkoop M, de Klerk BM, et al. Crepitus is a first indication of patellofemoral osteoarthritis (and not of tibiofemoral osteoarthritis). Osteoarthritis Cartilage. 2014; 22(5):631–638. PMID: 24583066.

3. Ho-Pham LT, Lai TQ, Mai LD, Doan MC, Pham HN, Nguyen TV. Prevalence of radiographic osteoarthritis of the knee and its relationship to self-reported pain. PLoS One. 2014; 9(4):e94563. PMID: 24722559.

4. Bae JH, Paik NH, Park GW, et al. Predictive value of painful popping for a posterior root tear of the medial meniscus in middle-aged to older Asian patients. Arthroscopy. 2013; 29(3):545–549. PMID: 23375180.

5. Dennis DA, Kim RH, Johnson DR, Springer BD, Fehring TK, Sharma A. The John Insall Award: control-matched evaluation of painful patellar Crepitus after total knee arthroplasty. Clin Orthop Relat Res. 2011; 469(1):10–17. PMID: 20706813.

6. Conrad DN, Dennis DA. Patellofemoral crepitus after total knee arthroplasty: etiology and preventive measures. Clin Orthop Surg. 2014; 6(1):9–19. PMID: 24605184.

7. Jackson JL, O'Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003; 139(7):575–588. PMID: 14530229.

8. Dajani KA, Stuart MJ, Dahm DL, Levy BA. Arthroscopic treatment of patellar clunk and synovial hyperplasia after total knee arthroplasty. J Arthroplasty. 2010; 25(1):97–103. PMID: 19106026.

9. Kim JH, Kim JR, Lee DH, Bang JY, Hong IT. Combined medial open-wedge high tibial osteotomy and modified Maquet procedure for medial compartmental osteoarthritis and patellofemoral arthritis of the knee. Eur J Orthop Surg Traumatol. 2013; 23(6):679–683. PMID: 23412178.

10. Andrish JT. Meniscal injuries in children and adolescents: diagnosis and management. J Am Acad Orthop Surg. 1996; 4(5):231–237. PMID: 10795058.

11. Cibere J, Bellamy N, Thorne A, et al. Reliability of the knee examination in osteoarthritis: effect of standardization. Arthritis Rheum. 2004; 50(2):458–468. PMID: 14872488.

12. Decary S, Ouellet P, Vendittoli PA, Desmeules F. Reliability of physical examination tests for the diagnosis of knee disorders: evidence from a systematic review. Man Ther. 2016; 26:172–182. PMID: 27697691.
13. Smith TO, Clark A, Neda S, et al. The intra- and inter-observer reliability of the physical examination methods used to assess patients with patellofemoral joint instability. Knee. 2012; 19(4):404–410. PMID: 21715175.

14. Cibere J, Zhang H, Thorne A, et al. Association of clinical findings with pre-radiographic and radiographic knee osteoarthritis in a population-based study. Arthritis Care Res (Hoboken). 2010; 62(12):1691–1698. PMID: 20665737.

15. Dupont JY. Synovial plicae of the knee: controversies and review. Clin Sports Med. 1997; 16(1):87–122. PMID: 9012563.
16. Neely LA, Kernohan WG, Barr DA, Mee CH, Mollan RA. Optical measurements of physiological patellofemoral crepitus. Clin Phys Physiol Meas. 1991; 12(3):219–226. PMID: 1934910.

17. Protopapas MG, Cymet TC. Joint cracking and popping: understanding noises that accompany articular release. J Am Osteopath Assoc. 2002; 102(5):283–287. PMID: 12033758.
18. Jiang CC, Liu YJ, Yip KM, Wu E. Physiological patellofemoral crepitus in knee joint disorders. Bull Hosp Jt Dis. 1993-1995; 53(4):22–26.
19. Prior J, Mascaro B, Shark LK, et al. Analysis of high frequency acoustic emission signals as a new approach for assessing knee osteoarthritis. Ann Rheum Dis. 2010; 69(5):929–930. PMID: 20413570.

20. Shark LK, Chen H, Goodacre J. Discovering differences in acoustic emission between healthy and osteoarthritic knees using a four-phase model of sit-stand-sit movements. Open Med Inform J. 2010; 4:116–125. PMID: 21379396.

21. Toreyin H, Jeong HK, Hersek S, Teague CN, Inan OT. Quantifying the consistency of wearable knee acoustical emission measurements during complex motions. IEEE J Biomed Health Inform. 2016; 20(5):1265–1272. PMID: 27305689.

22. Teague CN, Hersek S, Toreyin H, et al. Novel methods for sensing acoustical emissions from the knee for wearable joint health assessment. IEEE Trans Biomed Eng. 2016; 63(8):1581–1590. PMID: 27008656.

23. Bollen SR, Arvinte D. Snapping pes syndrome: a report of four cases. J Bone Joint Surg Br. 2008; 90(3):334–335. PMID: 18310756.
24. Cooper DE. Snapping popliteus tendon syndrome: a cause of mechanical knee popping in athletes. Am J Sports Med. 1999; 27(5):671–674. PMID: 10496589.
25. Demirag B, Ozturk C, Karakayali M. Symptomatic infrapatellar plica. Knee Surg Sports Traumatol Arthrosc. 2006; 14(2):156–160. PMID: 16059707.

26. Geeslin AG, LaPrade RF. Surgical treatment of snapping medial hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2010; 18(9):1294–1296. PMID: 20549187.

27. Kawchuk GN, Fryer J, Jaremko JL, Zeng H, Rowe L, Thompson R. Real-time visualization of joint cavitation. PLoS One. 2015; 10(4):e0119470. PMID: 25875374.

28. Brodeur R. The audible release associated with joint manipulation. J Manipulative Physiol Ther. 1995; 18(3):155–164. PMID: 7790795.
29. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990; 49(5):308–309. PMID: 2344210.

30. Unsworth A, Dowson D, Wright V. ‘Cracking joints’: a bioengineering study of cavitation in the metacarpophalangeal joint. Ann Rheum Dis. 1971; 30(4):348–358. PMID: 5557778.

31. Evans DW, Breen AC. A biomechanical model for mechanically efficient cavitation production during spinal manipulation: prethrust position and the neutral zone. J Manipulative Physiol Ther. 2006; 29(1):72–82. PMID: 16396734.

32. Reggars JW. The manipulative crack: frequency analysis. Australas Chiropr Osteopathy. 1996; 5(2):39–44. PMID: 17987137.
33. Roston JB, Haines RW. Cracking in the metacarpo-phalangeal joint. J Anat. 1947; 81(Pt 2):165–173. PMID: 17105029.
34. Nakayama A, Sugita T, Aizawa T, Takahashi A, Honma T. Incidence of medial plica in 3,889 knee joints in the Japanese population. Arthroscopy. 2011; 27(11):1523–1527. PMID: 21924860.

35. Lyu SR, Lee CC, Hsu CC. Medial abrasion syndrome: a neglected cause of knee pain in middle and old age. Medicine (Baltimore). 2015; 94(16):e736.
36. Stefancin JJ, Parker RD. First-time traumatic patellar dislocation: a systematic review. Clin Orthop Relat Res. 2007; 455:93–101. PMID: 17279039.
37. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007; 14(2):167–168. PMID: 17300941.

38. Segal A, Miller TT, Krauss ES. Fabellar snapping as a cause of knee pain after total knee replacement: assessment using dynamic sonography. AJR Am J Roentgenol. 2004; 183(2):352–354. PMID: 15269024.

39. Tensho K, Aoki T, Morioka S, Narita N, Kato H, Saito N. Snapping pes syndrome after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014; 22(1):192–194. PMID: 23263260.

40. Doherty M, Watt I, Dieppe PA. Localised chondrocalcinosis in post-meniscectomy knees. Lancet. 1982; 1(8283):1207–1210. PMID: 6122972.

41. Singh VK, Shah G, Singh PK, Saran D. Extraskeletal ossifying chondroma in Hoffa's fat pad: an unusual cause of anterior knee pain. Singapore Med J. 2009; 50(5):e189–e192. PMID: 19495507.
42. Paulos LE, Rosenberg TD, Drawbert J, Manning J, Abbott P. Infrapatellar contracture syndrome: an unrecognized cause of knee stiffness with patella entrapment and patella infera. Am J Sports Med. 1987; 15(4):331–341. PMID: 3661814.
43. Martin JR, Jennings JM, Watters TS, Levy DL, McNabb DC, Dennis DA. Femoral implant design modification decreases the incidence of patellar crepitus in total knee arthroplasty. J Arthroplasty. 2017; 32(4):1310–1313. PMID: 28012722.

44. Ranawat CS, White PB, West S, Ranawat AS. Clinical and radiographic results of attune and PFC sigma knee designs at 2-year follow-up: a prospective matched-pair analysis. J Arthroplasty. 2017; 32(2):431–436. PMID: 27600300.

45. Webb JE, Yang HY, Collins JE, Losina E, Thornhill TS, Katz JN. The evolution of implant design decreases the incidence of lateral release in primary total knee arthroplasty. J Arthroplasty. 2017; 32(5):1505–1509. PMID: 28089467.

46. Nam D, Barrack T, Nunley RM, Barrack RL. What is the frequency of noise generation in modern knee arthroplasty and is it associated with residual symptoms? Clin Orthop Relat Res. 2017; 475(1):83–90. PMID: 26762299.

47. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013; 21(9):577–579. PMID: 23996989.

48. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012; 36(12):2447–2456. PMID: 23052278.

49. Karataglis D, Papadopoulos P, Fotiadou A, Christodoulou AG. Snapping knee syndrome in an athlete caused by the semitendinosus and gracilis tendons: a case report. Knee. 2008; 15(2):151–154. PMID: 18262790.

50. Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005; 12(6):458–460. PMID: 16006128.

51. Magi M, Branca A, Bucca C, Langerame V. Hoffa disease. Ital J Orthop Traumatol. 1991; 17(2):211–216. PMID: 1797732.
Table 1
Comparison of Physiological and Pathological Sources of Noise around the Knee
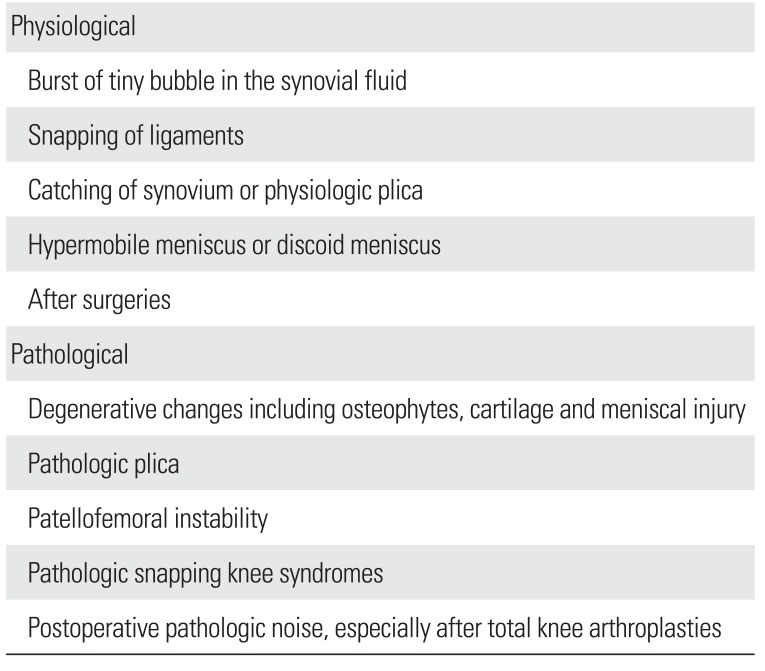
Table 2
Factors Affecting the Risk of Patellofemoral Crepitus or Clunk after Total Knee Arthroplasty




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download