Abstract
Subcutaneous fat necrosis (SFN) in newborns is a rare disease that affects infants in the first few weeks after birth. The lesion involves the back, buttocks, thighs, arms, and cheeks and it appears as a subcutaneous nodule in firm, well-defined, purple-red manifestation. It is a self-limited disorder and follows an uncomplicated course, but serious complications may occur such as thrombocytopenia, hypoglycemia, hypertriglyceridemia, and hypercalcemia. I am reporting a case of ultrasonographic and MR imaging findings of SFN in a 35–day-old girl with hypercalcemia and medullary nephrocalcinosis.
Subcutaneous fat necrosis (SFN) in newborns is a rare, temporary, self-limited disorder of subcutaneous tissue. It affects infants in the first few weeks after birth. Clinical manifestation includes firm to rubbery, well-defined, purple-red subcutaneous nodules and plaques on the back, buttocks, thighs, arms and cheeks (1). While the underlying cause of SFN is unknown, perinatal complications such as perinatal asphyxia, meconium aspiration, hypoglycemia, and hypothermia are suggested as possible risk factors (2). Major acute complications of SFN are hypercalcemia, hypertriglyceridemia, and transient thrombocytopenia, especially hypercalcemia can result in a fatal outcome (3). Although there are a few published cases of radiologic findings of SFN in newborns (245), reports of radiologic findings of nephrocalcinosis from SFN are rare (6). This study presents an additional case of SFN of a newborn and describes ultrasonographic and MR imaging of subcutaneous lesion and renal ultrasonographic findings.
The patient was a 4400-g female infant born at 39 weeks of gestation. The mother was 21 years old and the baby was born by normal vaginal delivery with cephalic presentation, but aspirated meconium. Apgar scores were 7 at 1 minute and 8 at 5 minutes. Since cyanosis and respiratory distress occurred during feeding, the patient was taken to a hospital two days after birth. Laboratory data were as follows; hemoglobin of 16.7 g/dL, hematocrit of 52.4%, platelet count of 137 × 103/µL, elevated liver function tests with aspartate aminotransferase of 494 IU (normal in newborn, 60 ± 8), alanine aminotransferase of 307 IU (normal in newborn, 20 ± 3), total calcium of 7.4 mg/dL (normal, 8.5–10.5), and phosphate of 3.2 mg/dL (normal, 2.5–4.5). Hepatomegaly was revealed in abdominal ultrasonography. The pediatrician presumed that the patient had neonatal hepatitis and treated the patient conservatively. On three-day follow-up, liver function tests returned to normal range.
Thirty five days after birth, the patient revisited for follow-up abdominal ultrasonography and was presented with palpable, large, firm and non-movable masses on the right cheek, submandibular area, shoulders, axilla, buttock, and back two weeks before the revisit. Overlying skin was violaceous and erythematous. Ultrasonography was conducted for palpable lesions and revealed lobulated hyperechoic lesions in subcutaneous layer of the right cheek, submandibular area, shoulders, axilla, buttock, and back. Lesions were separated from dermis (Fig. 1A) and neither calcification nor cystic portion was detected. On color Doppler ultrasonography, there was no vascularity in hyperechoic masses (Fig. 1B). Follow-up abdominal ultrasonography revealed improved hepatomegaly, but revealed bilateral medullary nephrocalcinosis (Fig. 1C). Serum calcium level was elevated to 11.9 mg/dL (normal, 8.5–10.5) and ionized calcium was 1.53 mg/dL (normal, 0.9–1.3). MR imaging of the pelvis revealed well-defined masses in subcutaneous layer of buttocks and inguinal areas and signal intensities of lesions were hyperintense compared with muscle on T1- and T2-weighted MR images (Fig. 1D, E). Percutaneous core needle biopsy was conducted at the right buttock using an 18-gauge automated gun. Microscopically, the lesion consisted of necrotic adiopocytes and inflammatory reaction with presence of giant cells, consistent histological features with SFN. During the 10-week follow-up period, palpable lesions gradually resolved, and total calcium level was decreased to 10.7 mg/dL. At 12-week follow-up abdominal ultrasonography, medullary nephrocalcinosis were improved. We were unable to check serum calcium levels because the patient did not revisit for follow-up.
SFN in newborns is an uncommon disease affecting full-term or post-term newborns and appears in the first few weeks after birth. It is associated with perinatal complications such as perinatal asphyxia, meconium aspiration, birth trauma, hypoglycemia, and hypothermia or maternal disease such as preeclamsia and gestational diabetes (123). Characteristic lesions are composed of firm to rubbery, circumscribed, non-tender and mobile nodules and plaques and overlying skin may be purple-red, erythematous, or normal in appearance (14). The back, buttocks, thighs, arms and cheeks are common sites of involvement (1).
SFN has positive prognosis to regress spontaneously within several weeks to six months (13). However, there are significant complications such as hypercalcemia, thrombocytopenia, hypoglycemia, and hypertriglyceridemia (137), so the goal of treatment is prevention and management of complications. Incidence of hypercalcemia and thrombocytopenia have been reported at 28–56% (17) and 6% (7) respectively. Incidence of other complications has not been noted because other complications have been reported in case reports of a limited number of patients and they may be influenced by sepsis or gestational diabetes (3). Hypercalcemia is the most serious complication of SFN because of potential lethal effects on cardiovascular and the renal system. Symptoms of hypercalcemia include cardiac arrhythmias, nausea, vomiting, constipation, paralytic ileus, renal impairment, hypotonia, and failure to thrive. Nephrocalcinosis may occur following persistent hypercalcemia and results in renal colic, polyuria, and polydipsia (8). Hypercalcemia usually occurs from one-six months when skin lesions begin to resolve so patients with SFN should have serum calcium levels regularly monitored for up to six months after occurrence of skin lesions (23). Pathogenesis of hypercalcemia in SFN is unknown, but the most reasonable hypothesis is that excessive production of 1,25(OH)2D3 from SFN lesions will stimulate intestinal calcium uptake (9).
In a previously reported case, ultrasonography of the SFN revealed lobulated well-defined hyperechoic nodules with increased vascularity and vessels passing through nodules (2). However, in this case, vascularity was not distinct on Doppler study. On CT scans, lesions of SFN were localized to subcutaneous tissues just below the dermis and revealed two different imaging findings. One type was discrete subcutaneous nodules and the other type was diffuse subcutaneous thickening (5). Anderson et al. (4) reported MR images of SFN revealed hypointense lesions compared with subcutaneous fat on T1- and T2-weighted images, but, in their report, signal intensity was compared with subcutaneous fat, so signal intensity was higher than that for muscle. Srinath and Cohen (2) reported that signal intensity of SFN was compared with muscle and signal intensity was higher than that for muscle and this case also demonstrated similar MR imaging findings. Previously reported cases of renal ultrasonographic findings of SFN with hypercalcemia were rare and revealed nephrocalcinosis and nephrolithiasis (6). Nephrolithiasis disappeared after several months, and nephrocalcinosis lasted longer than nephrolithiasis.
Differential diagnosis includes soft tissue tumors of a newborn, such as rhabdomyosarcomas, fibromatoses, hemangiomas, and sclerema neonatorum. Rhabdomyosarcoma is solitary and involves a muscle. Fibromatoses that may affect children include infantile myofibromatosis and desmoid fibromatosis. Infantile myofibromatosis may affect newborns, but these typically involve muscle. Desmoid fibromatoses may be superficial lesions, but usually affect somewhat older children. Hemangioma may be multiple, but usually more deeply located than SFN. Sclerema neonatorum arises in a newborn and involves subcutaneous tissue, but is typically a localized lesion (10).
In conclusion, SFN in a newborn is a rare clinical entity with distinctive radiologic features. Treatment must be conservative because it has positive prognosis if associated hypercalcemia is well managed and surgical treatments are avoided. Therefore, patients with SFN need monitoring of serial serum calcium levels and observation of symptoms and signs of hypercalcemia for up to six months after occurrence of skin lesions.
Figures and Tables
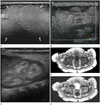 | Fig. 1Subcutaneous fat necrosis of a newborn in a 35-day-old girl.
A. Ultrasonography of the right buttock reveals lobulated, hyperechoic mass (arrows) in the subcutaneous layer separated from dermis. Similar findings are observed in the right cheek, submandibular area, shoulders, axilla, and back (not shown).
B. No vascularity is detected on color Doppler ultrasonography.
C. Renal ultrasonography reveals increased echogenicity of renal medulla, suggesting medullary nephrocalcinosis.
D, E. Axial T1-weighted (D) and T2-weighted (E) pelvis MR images after eight days of ultrasonography reveal hyperintense lesions (arrows) compared with the muscle in the subcutaneous layer of buttocks and inguinal areas.
|
References
1. Burden AD, Krafchik BR. Subcutaneous fat necrosis of the newborn: a review of 11 cases. Pediatr Dermatol. 1999; 16:384–387.
2. Srinath G, Cohen M. Imaging findings in subcutaneous fat necrosis in a newborn. Pediatr Radiol. 2006; 36:361–363.

3. Tran JT, Sheth AP. Complications of subcutaneous fat necrosis of the newborn: a case report and review of the literature. Pediatr Dermatol. 2003; 20:257–261.

4. Anderson DR, Narla LD, Dunn NL. Subcutaneous fat necrosis of the newborn. Pediatr Radiol. 1999; 29:794–796.

5. Norton KI, Som PM, Shugar JM, Rothchild MA, Popper L. Subcutaneous fat necrosis of the newborn: CT findings of head and neck involvement. AJNR Am J Neuroradiol. 1997; 18:547–550.
6. Gu LL, Daneman A, Binet A, Kooh SW. Nephrocalcinosis and nephrolithiasis due to subcutaneous fat necrosis with hypercalcemia in two full-term asphyxiated neonates: sonographic findings. Pediatr Radiol. 1995; 25:142–144.

7. Mahé E, Girszyn N, Hadj-Rabia S, Bodemer C, Hamel-Teillac D, De Prost Y. Subcutaneous fat necrosis of the newborn: a systematic evaluation of risk factors, clinical manifestations, complications and outcome of 16 children. Br J Dermatol. 2007; 156:709–715.

8. Borgia F, De Pasquale L, Cacace C, Meo P, Guarneri C, Cannavo SP. Subcutaneous fat necrosis of the newborn: be aware of hypercalcaemia. J Paediatr Child Health. 2006; 42:316–318.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download