Abstract
A 34-year-old female patient underwent uterine artery embolization (UAE) to control massive postpartum hemorrhage. The interventional radiologist was not informed of the patient's significant history of uterine myoma. Although no significant signs of complications or “red flags” were observed during the procedure, follow-up computed tomography performed four weeks later revealed evidence of a large, globe-like fluid collection with air bubbles in the uterus. The finding and pathology was initially diagnosed as uterine necrosis, which led not to interventional percutaneous drainage; instead, dilation and curettage with resectoscope was performed. The surgical and pathological diagnosis was “expulsion of pyomyoma in the uterine cavity.” Awareness and precise knowledge of imaging findings of pyomyoma and uterine necrosis are important for early diagnosis and treatment of UAE-related complications.
Postpartum hemorrhage (PPH) is one of the major causes of maternal mortality. Uterine artery embolization (UAE) is regarded a useful and reliable procedure to control and manage PPH. However, it has been reported that there are possible complications arising from this procedure, including disruption of the patients' normal menstrual cycles, infertility, pyometra and/or necrosis of the uterus and/or bladder.
Herein, we are reporting on a rare case of pyomyoma, initially misdiagnosed as uterine necrosis after UAE. Pyomyoma was ultimately diagnosed but not until later, after successful management by dilation and curettage (D&C), with resectoscope.
For this study, approval from the Institutional Review Board of our institution was obtained. Medical records were retrospectively reviewed, so the requirement for obtaining informed consent was waived.
A 34-year-old female patient was transferred to our hospital for PPH after normal spontaneous vaginal delivery at 39 weeks of her second pregnancy. The estimated blood loss was approximately 1500 mL. Before transfer, she was managed conservatively, including intravenous administration of oxytocin, transfusion and vaginal gauze packing, but her condition would not stabilize. She had blood pressure of 80/60 mm Hg, pulse of 105 per minute and hemoglobin of 11.0 g/dL.
Obstetrician performed an ultrasound which showed two uterine myomas in the anterior and posterior walls of uterus. The diagnosis by obstetrician was hematometra and myomas in the postpartum uterus. However, the information regarding the presence of uterine myoma was not conveyed to the department of interventional radiology.
An emergency UAE was performed for massive PPH by a fellowship-trained interventional radiologist with eleven-months on-the-job experience. Selective angiography of both internal iliac arteries performed from the femoral approach showed that her uterine arteries were engorged, but without any sign of extravasation of blood (Fig. 1A). No definite evidence of uterine myoma was found on selective angiography of any of the uterine arteries.
A selective UAE with gelatin sponge particles (GSP) (Cali-Gel®; Alicon Pharm SCI&TEC Co., Ltd., Hangzhou, China) was performed with the catheter tip placed beyond the origin of the cervico-vaginal branch on each side, using a microguidewire (Chikai V; Asahi Intecc, Hanoi, Vietnam) and a micro-catheter (Parkway; Asahi Intecc, Pathum Thani, Thailand), until complete cessation of blood flow. Complete cessation of blood flow refers to stagnation of contrast agent in the ascending branch of the uterine artery for ten cardiac beats. While three vials of GSP (560–710 micro: one vial, 710–1000 micro: one vial, 1000–1400 micro: one vial) were used for left UAE, two and a quarter vials of GSP (560–710 micro: one vial, 710–1000 micro: one vial, 1000–1400: 1/4 vial) were used for right UAE. Based on the knowledge gained from his past experience, the interventional radiologist decided to gradually increase the size of GSP while performing the UAE. In his past clinical experience, he had used relatively larger embolic materials for occlusions of uterine arteries in the early phase, which unfortunately resulted in a redo procedure and ended up requiring surgical re-intervention.
The interventional radiologist performed supra-renal aortography each before and after UAE. The supra-renal aortography performed after the embolization recognized both ovarian arteries as collateral pathways. The procedure was terminated as the arteries were not engorged to sufficient size to permit continuation of the procedure, meaning no additional embolization was necessary.
No technical failure or complications resulted from the interventional procedure, which successfully stabilized the patient. Her blood pressure was 111 mm Hg, pulse was 85 beats per minute and hemoglobin was 11.3 g/dL. The post-procedural ultrasonography performed by the attending obstetrician detected hematometra and several remaining uterine myomas (Fig. 1B). As a result, she was discharged home from hospital and said to be improved.
After a month, the patient returned to the hospital and was admitted as an in-patient through the emergency room with fever, abdominal pain and vaginal discharge with a foul odor. Abdomino-pelvic computed tomography (CT) showed fluid collection with air bubbles in the uterus (Fig. 1C).
The patient was observed and her course of care was supervised in a hospital setting. She was treated with intravenous administration of antibiotics such as metronidazole (Flagyl; JW Life Science Co., Ltd., Dangjin, Korea), cefotetan (Yamatetan; Jeil Pharmaceutical Co., Ltd., Seoul, Korea) and tetracycline (Doxycycline; Kukje Pharmaceutical Co., Ltd., Seongnam, Korea). While suffering from persisting symptoms, she had blood pressure 108/52 mm Hg, pulse 113 beats per minute, temperature 38.7℃, leukocyte count 8420/µL, C-reactive protein 93.6 mg/L and an erythrocyte sedimentation rate 54 mm/hour. A culture of blood samples showed negative results. Culture of the cervix performed the day after admission showed corynebacterium amycolatum, pseudomonas monteilii, and α-streptococcus. No signs or symptoms of upper respiratory infection or urinary tract infection appeared in the patient. In addition, there were no remarkable symptoms of headache or diarrhea, which might have suggested that infection occurred in, or had spread to, body parts other than the uterus.
On the third day of admission, there were no specific signs except fever, and the amount of vaginal discharge was reduced. However, despite the use of antibiotics, intermittent fever lingered. The attending obstetrician considered the CT finding as consistent with uterine abscess, and scheduled a D&C with resectoscope to be done two days later. As an alternative measure, the treating obstetrician planned to perform a hysterectomy if the D&C failed, or the patient's condition did not improve.
On the fourth day, obstetrician consulted with the interventional radiologist on percutaneous drainage before obstetric surgery. The interventional radiologist opined that the CT findings appeared to be most consistent with uterine necrosis, which could not be drained by insertion of drainage catheters. Therefore, surgical intervention consisting of hysterectomy seemed to be the remaining viable treatment option.
On the fifth day, the genitourinary radiologist reviewed and re-interpreted the CT findings and concluded that the CT findings were likely indicative of UAE-related changes (necrotic debris) and several infarcted uterine myomas. A D&C with resectoscope was performed by obstetrician on the same day. The obstetrician identified a large amount of necrotic debris on hysteroscopy, and successfully performed the D&C with resectoscope (Fig. 1D). The necrotic debris was examined by surgical pathology, and confirmed as pyomyoma. A cervical culture performed three days after the D&C showed no evidence of growth for the ensuing 72 hours.
The patient was discharged a week after the surgery as her condition was stabilized. The patient's menstrual cycle returned to normal from the subsequent one. Follow-up ultrasonography performed nine months later confirmed myoma without any notable findings in uterus (Fig. 1E).
Although UAE for PPH is generally considered a safe and effective procedure, it may result in several significant medical complications such as uterine necrosis and pyomyoma. CT findings of uterine necrosis and pyomyoma share similar imaging manifestations with each other, but major differences between them exist as follows. Case reports that have been published to date describe that CT or magnetic resonance appearances of uterine necrosis reveal central necrotic portions and peripheral enhancement of myometrium (one case) and central necrotic portions without enhancement of myometrium (four cases) (123). On the contrary, CT findings arising from pyomyoma show central necrotic portions without decrease in the enhancement of myometrium (two cases) and heterogeneous, multi-lobular mass with low attenuation and the presence of gas in the mass (one case) (456). Most of the pyomyoma cases show persistent enhancement of myometrium, thought to represent the initial diagnostic clue for pyomyoma, consistent with our case.
A retrospective review of images reveals that the opening of the cervix was caused by space-occupying lesion of unknown cause in the uterine cavity, which proved to be the second clue for pyomyoma. Case reports in the literature describe the possibility of vaginal expulsion of fibroid after UAE (7). In addition, myoma commonly expand and enlarge during pregnancy (8). Given these two factors, the interventional radiologist should be aware of the possibility of myoma and subsequent expulsion.
In conclusion, it would seem that the aggregate of the elements discussed above point to the conclusion that the patient should have been diagnosed as afflicted with pyomyoma, not uterine necrosis.
We suggest that information regarding the presence of uterine myoma be conveyed to the interventional radiologist before UAE. In this case and as we have seen, the interventional radiologist was not advised of the presence of uterine myoma by the obstetrician. Accordingly, the physician could not anticipate the possible presence or complications arising from combined uterine myoma before UAE. We are mindful that on uterine artery angiography, myoma is usually shown as distortion and enlargement of the uterine arteries, but this was not the case here. As a result, the possibility of pyomyoma was not considered for some time, in turn postponing the delivery of appropriate and necessary physician-supervised care.
In most cases of documented uterine necrosis, hysterectomy is the treatment option of choice. No studies were conducted on uterine necrosis after UAE which was treated with intravenous antibiotics or hysteroscopic surgical drainage procedures (9). Although pyomyoma is usually treated with hysterectomy, it is not impossible to treat with hysteroscopic surgical drainage (10).
For interventional radiologists, it is critical to be notified of the existence of myoma. If the interventional radiologist is uncertain about the presence of uterine myoma, he should consider performing pre-procedural ultrasonography personally. After all, communication between the obstetrician and the interventional radiologist is the key.
It is important to determine whether patient is suffering from post-embolization syndrome (PES) or pyomyoma. PES refers to syndrome consisting of fever, abdominal pain, nausea and vomiting which subsides within a few days. Exclusion of PES and presence of intra-uterine infection are supposed on the basis of one or more of the following: presence of foul-smelling vaginal discharge, clinical course of the initial symptoms, clinical improvement with therapy and presence of high-grade leukocytosis (10).
In case of patients with myoma who have symptoms of abdominal pain and sepsis after UAE, the possibility of pyomyoma should be considered so that patients can be treated in an appropriate and a timely manner.
Furthermore, precise and specialized knowledge and understanding of radiographic imaging findings and clinical courses of pyomyoma and uterine necrosis are essential for early diagnosis and treatment of UAE-related complications.
Figures and Tables
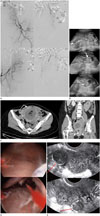 | Fig. 1A 34-year-old female patient with pyomyoma after UAE for postpartum hemorrhage, misdiagnosed as uterine necrosis.
A. Angiography of both internal iliac arteries and both uterine arteries was performed on each side. Both uterine arteries (arrows) are engorged, but no active bleeding is detected on angiogram. There is no definitive evidence of myoma on angiography.
B. Images of transvaginal uterine ultrasonography 4 days after UAE. Ultrasonography shows hypoechoic area (curved arrow) within uterine cavity, suggesting hematometra. There are also several heterogeneous hyperechoic myomas (arrows) in uterus endometrium and myometrium.
UAE = uterine artery embolization
C. Images of abdominopelvic computed tomography performed due to fever, abdominal pain and vaginal discharge a month after UAE. Fluid collection with air bubbles and peripheral enhancement in uterine myoma, which is measured about 12.7 × 8.2 × 7.2 cm, is seen in the enlarged uterine cavity. We misdiagnosed this as uterine necrosis, which should have been identified as pyomyoma. Peripheral myometrial enhancement (curved arrow) was diagnostic clue for pyomyoma. In addition, opening of cervix (arrow) by expulsed myoma was diagnostic evidence for pyomyoma.
UAE = uterine artery embolization
D. Images of hysteroscope performed by obstetrician. Myoma is observed in anterior wall of uterus and suppurative necrotic debris (arrow) was found in uterine cavity on hysteroscope.
E. Images of transvaginal uterine ultrasonography nine months after dilation and curettage with resectoscope. Two myomas measuring 2.0 × 1.9 cm and 1.6 × 1.2 cm are noted in myometrium of uterus (arrows), which are measured 4.8 × 2.9 cm and 3.3 × 2.8 cm respectively, on uterine ultrasonography previously performed by obstetrician before UAE. Except for that, there is no remarkable finding in uterus.
UAE = uterine artery embolization
|
References
1. Courbiere B, Jauffret C, Provansal M, Agostini A, Bartoli JM, Cravello L, et al. Failure of conservative management in postpartum haemorrhage: uterine necrosis and hysterectomy after angiographic selective embolization with gelfoam. Eur J Obstet Gynecol Reprod Biol. 2008; 140:291–293.

2. Tseng JJ, Ho JY, Wen MC, Hwang JI. Uterine necrosis associated with acute suppurative myometritis after angiographic selective embolization for refractory postpartum hemorrhage. Am J Obstet Gynecol. 2011; 204:e4–e6.

3. Melenhorst M, Hehenkamp W, de Heer K, Ferco HB. CT features in uterine necrosis of unknown cause: a case report. Clin Imaging. 2014; 38:543–546.

4. Karcaaltincaba M, Sudakoff GS. CT of a ruptured pyomyoma. AJR Am J Roentgenol. 2003; 181:1375–1377.

5. Iwahashi N, Mabuchi Y, Shiro M, Yagi S, Minami S, Ino K. Large uterine pyomyoma in a perimenopausal female: a case report and review of 50 reported cases in the literature. Mol Clin Oncol. 2016; 5:527–531.

6. Obele CC, Dunham S, Bennett G, Pagan J, Sung LY, Charles HW. A case of pyomyoma following uterine fibroid embolization and a review of the literature. Case Rep Obstet Gynecol. 2016; 2016:9835412.

7. Martins JG, Gaudenti D, Crespo F, Ganesh D, Verma U. Uncommon complication of uterine artery embolization: expulsion of infarcted myoma and uterine sepsis. Case Rep Obstet Gynecol. 2016; 2016:8695318.

8. De Vivo A, Mancuso A, Giacobbe A, Savasta LM, De Dominici R, Dugo N, et al. Uterine myomas during pregnancy: a longitudinal sonographic study. Ultrasound Obstet Gynecol. 2011; 37:361–365.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download