Abstract
Purpose
The aim of this study was to accurately evaluate the significance and correlation between the clinical severity and the morphologic feature of respiratory muscles in patients with emphysema as noted using computed tomography (CT).
Materials and Methods
The cross sectional area (CSA) and attenuation of respiratory muscles in the patients with emphysema (n = 71) were subsequently retrospectively reviewed. The clinical severity for the patients was determined by the value of the actual forced expiratory volume in 1 second/forced vital capacity at the pulmonary function test (PFT). The correlation between the CT measurements with visual assessment of emphysema (VAE), and the PFT values were completed and recorded. The multiple linear regression analysis of each CT measurement on the VAE and PFT values was used to determine the most affective parameters among the recorded and identified CT measurements.
Results
The CSA of the pectoralis major (p = 0.002) and subsequently the serratus anterior (p = 0.011) were found to be lower in patients with emphysema than as compared to those in the control group. The CSA and the attenuation of respiratory muscles remained significant for its relation for the VAE and PFT values. As noted, both the VAE and PFT values were mostly contributed by the CSA and attenuation of serratus anterior and attenuation of diaphragm crus among all respiratory muscles.
Go to : 
References
1. Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J Suppl. 2003; 46:41s–51s.

2. Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997; 337:1799–1806.

3. Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, et al. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol. 2002; 92:1205–1213.
4. Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, et al. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol. 1998; 274:L527–L534.
5. Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991; 325:917–923.

8. Ishikawa S, Hayes JA. Functional morphometry of the diaphragm in patients with chronic obstructive lung disease. Amer Rev Resp Dis. 1973; 108:135–138.
9. McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. a cross-sectional study. Ann Am Thorac Soc. 2014; 11:326–334.

10. Park MJ, Cho JM, Jeon KN, Bae KS, Kim HC, Choi DS, et al. Mass and fat infiltration of intercostal muscles measured by CT histogram analysis and their correlations with COPD severity. Acad Radiol. 2014; 21:711–717.

11. Huang YS, Hsu HH, Chen JY, Tai MH, Jaw FS, Chang YC. Quantitative computed tomography of pulmonary emphysema and ventricular function in chronic obstructive pulmonary disease patients with pulmonary hypertension. Korean J Radiol. 2014; 15:871–877.

12. Yoon SH, Goo JM, Jung J, Hong H, Park EA, Lee CH, et al. Computer-aided classification of visual ventilation patterns in patients with chronic obstructive pulmonary disease at two-phase xenon-enhanced CT. Korean J Radiol. 2014; 15:386–396.

13. Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015; 277:192–205.

14. Smith BM, Austin JH, Newell JD Jr, D'Souza BM, Rozensh-tein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014; 127:94. .e7–23.

15. Nakayama Y, Awai K, Funama Y, Hatemura M, Imuta M, Nakaura T, et al. Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology. 2005; 237:945–951.

16. Rho M, Spitznagle T, Van Dillen L, Maheswari V, Oza S, Prather H. Gender differences on ultrasound imaging of lateral abdominal muscle thickness in asymptomatic adults: a pilot study. PM R. 2013; 5:374–380.

17. Sharp JT, Danon J, Druz WS, Goldberg NB, Fishman H, Mach-nach W. Respiratory muscle function in patients with chronic obstructive pulmonary disease: its relationship to disability and to respiratory therapy. Am Rev Respir Dis. 1974; 110:154–168.
18. Rochester DF, Braun NM. Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985; 132:42–47.
19. Cassart M, Pettiaux N, Gevenois PA, Paiva M, Estenne M. Effect of chronic hyperinflation on diaphragm length and surface area. Am J Respir Crit Care Med. 1997; 156:504–508.

20. Kim SS, Seo JB, Lee HY, Nevrekar DV, Forssen AV, Crapo JD, et al. Chronic obstructive pulmonary disease: lobe-based visual assessment of volumetric CT by using standard images–comparison with quantitative CT and pulmonary function test in the COPDGene study. Radiology. 2013; 266:626–635.

21. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33:1165–1185.

22. Caron MA, Debigaré R, Dekhuijzen PN, Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol. 2009; 107:952–961.

23. Nishimura Y, Tsutsumi M, Nakata H, Tsunenari T, Maeda H, Yokoyama M. Relationship between respiratory muscle strength and lean body mass in men with COPD. Chest. 1995; 107:1232–1236.

24. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370:797–799.

Go to : 
 | Fig. 1.Sample CT scans used to measure cross sectional area of respiratory muscles. A. The cross sectional area of PM (red) and PN (blue) are measured at the level of the claviculomanubrial joint. B. The cross sectional area of ITC (purple), SA (green), and LD (yellow) are measured at the level of right inferior pulmonary vein. C. The cross sectional area of DC (orange) is measured at the retrocrural area at the level of origin of the celiac trunk. DC = diaphragm crus, ITC = intercostalis, LD = latissimus dorsi, PM = pectoralis major, PN = pectoralis minor, SA = serratus anterior |
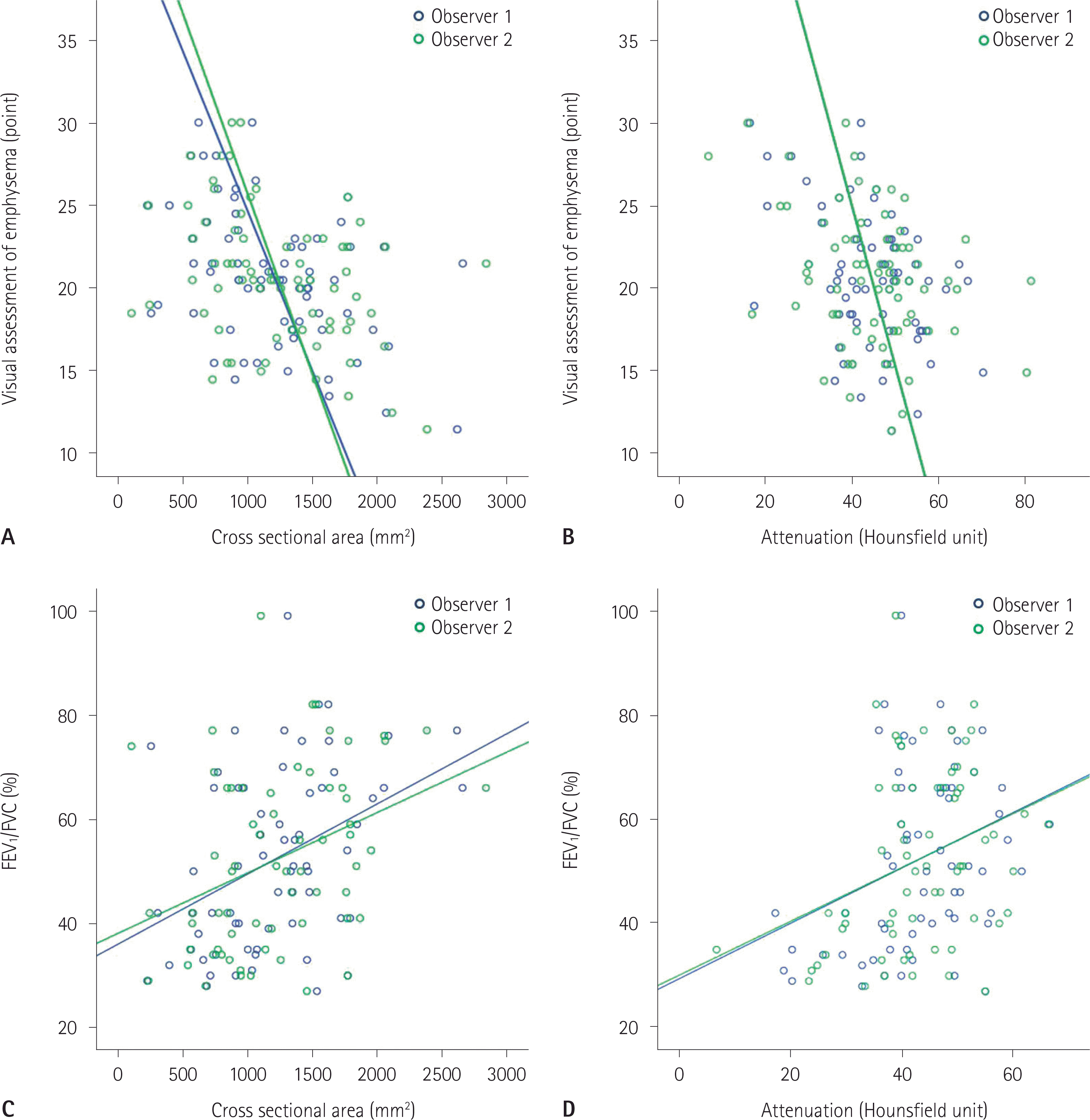 | Fig. 2.Correlation of CT measurements of serratus anterior according to visual assessment of emphysema (A, B) and FEV1/FVC (C, D). FEV1/FVC = forced expiratory volume in 1 second/forced vital capacity |
Table 1.
Characteristics of 71 Male Patients with Emphysema
| Total patient number | 71 |
|---|---|
| Age (years) | 69.6 ± 10.2 |
| BMI (kg/m2) | 21.7 ± 3.3 |
| GOLD stage | |
| Normal | 12 |
| Mild | 14 |
| Moderate | 16 |
| Severe | 22 |
| Very severe | 7 |
| VAE∗ | |
| 0% (6 point) | 0 |
| 1–5% (7–11 point) | 1 |
| 6–25% (12–17 point) | 17 |
| 26–50% (18–23 point) | 37 |
| 51–75% (24–29 point) | 14 |
| > 75% (30–36 point) | 2 |
Table 2.
CT Measurements of Respiratory Muscles
| Emphysema (n = 71) | Control (n = 24) | p-Value | |
|---|---|---|---|
| Age (years) | 69.6 ± 10.2 | 68.8 ± 13.3 | 0.249 |
| BMI (kg/m2) | 21.7 ± 3.3 | 20.5 ± 3.3 | 0.383 |
| Height (m) | 1.66 ± 0.06 | 1.69 ± 0.07 | 0.108∗ |
| CSA (cm2) | |||
| Pectoralis major | 21.9 ± 5.9 | 26.5 ± 6.8 | 0.002∗ |
| Pectoralis minor | 7.7 ± 2.1 | 7.9 ± 2.2 | 0.566 |
| Intercostal | 1.0 (0.2–3.2) | 0.8 (0.3–2.3) | 0.155† |
| Serratus anterior | 12.1 ± 5.0 | 15.4 ± 5.8 | 0.011∗ |
| Latissimus dorsi | 11.7 (3.1–23.6) | 13.0 (6.0–24.7) | 0.136† |
| Diaphragm crus | 2.7 ± 1.0 | 2.8 ± 1.3 | 0.644 |
| Attenuation | |||
| Pectoralis major | 47.4 ± 10.3 | 45.1 ± 8.0 | 0.736 |
| Pectoralis minor | 45.9 ± 8.6 | 44.4 ± 6.4 | 0.149 |
| Intercostal | 2.3 ± 14.7 | 2.6 ± 17.4 | 0.320 |
| Serratus anterior | 39.8 (9.5–59.8) | 42.3 (25.5–59) | 0.300† |
| Latissimus dorsi | 27.0 (−14.5–52.5) | 31.0 (−0.2–51.5) | 0.222† |
| Diaphragm crus | 32.2 ± 10.6 | 35.8 ± 7.8 | 0.138 |
Table 3.
Repeatability of CT Measurements of Respiratory Muscles
Table 4.
Pearson Correlation Coefficient (r) between CT Measurements and FEV1/FVC
| Variables | VAE (p-Value) | FEV1/FVC (p-Value) |
|---|---|---|
| CSA | ||
| Pectoralis major | –0.210 (0.078) | –0.052 (0.227) |
| Pectoralis minor | –0.144 (0.232) | –0.105 (0.904) |
| Intercostalis | –0.245 (0.040)∗ | –0.064 (0.613) |
| Serratus anterior | –0.389 (0.001)∗ | –0.390 (0.001)∗ |
| Latissimus dorsi | –0.343 (0.003)∗ | –0.285 (0.022)∗ |
| Diaphragm crus | –0.138 (0.250) | –0.083 (0.513) |
| Attenuation | ||
| Pectoralis major | –0.330 (0.005) | 0.207 (0.098) |
| Pectoralis minor | –0.408 (< 0.001)∗ | 0.271 (0.029)∗ |
| Intercostalis | –0.328 (0.005)∗ | 0.210 (0.094) |
| Serratus anterior | –0.376 (0.001)∗ | 0.334 (0.007)∗ |
| Latissimus dorsi | –0.138 (0.251) | 0.155 (0.217) |
| Diaphragm crus | –0.329 (0.005)∗ | 0.376 (0.002)∗ |
Table 5.
Relationship of CT Measurements of Respiratory Muscles to VAE and FEV1/FVC in Patients with Emphysema, Respectively
| Respiratory Muscles | VAE | FEV1/FVC | ||||||
|---|---|---|---|---|---|---|---|---|
| CSA | Attenuation | CSA | Attenuation | |||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Simple regression | ||||||||
| Pectoralis major | –0.202 cm2 | 0.247 | –0.130 | 0.007∗ | 0.229 cm2 | 0.629 | 0.256 | 0.238 |
| Pectoralis minor | –0.048 cm2 | 0.863 | –0.192 | 0.001∗ | –0.408 cm2 | 0.736 | 0.428 | 0.083 |
| Intercostalis | –1.197 cm2 | 0.220 | –0.092 | 0.004∗ | 0.911 cm2 | 0.836 | 0.211 | 0.142 |
| Serratus anterior | –0.333 cm2 | 0.004∗ | –0.172 | < 0.001∗ | 1.444 cm2 | 0.004∗ | 0.524 | 0.013∗ |
| Latissimus dorsi | –0.321 cm2 | 0.026∗ | –0.044 | 0.195 | 1.262 cm2 | 0.047∗ | 0.134 | 0.360 |
| Diaphragm crus | –0.154 cm2 | 0.770 | –0.115 | 0.014∗ | 0.661 cm2 | 0.771 | 0.551 | 0.007∗ |
| Stepwise multiple regression | ||||||||
| Serratus anterior | –0.312 cm2 | 0.001∗ | –0.122 | 0.046∗ | 1.760 cm2 | < 0.001∗ | 0.419 | 0.040∗ |
| Diaphragm crus | – | – | –0.111 | 0.012∗ | – | – | 0.492 | 0.012∗ |
| Pectoralis minor | – | – | –0.164 | 0.014∗ | – | – | – | – |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download