Abstract
Artificial intelligence is expected to influence clinical practice substantially in the foreseeable future. Despite all the excitement around the technology, it cannot be denied that the application of artificial intelligence in medicine is overhyped. In fact, artificial intelligence for medicine is presently in its infancy, and very few are currently in clinical use. To best leverage the potential of this technology to improve patient care, clinicians need to see beyond the hype, as the guidance and leadership of medical professionals are critical in this matter. To this end, medical professionals must understand the underlying technological basics of artificial intelligence, as well as the methodologies of its proper clinical validation. They should also have an impartial, complete view of the capabilities, pitfalls, and limitations of the technology and its use in healthcare. The present article provides succinct explanations of these matters and suggests further reading materials (peer-reviewed articles and web pages) for medical professionals who are unfamiliar with artificial intelligence.
Go to : 
References
1. Lee JG, Jun S, Cho YW, Lee H, Kim GB, Seo JB, et al. Deep learning in medical imaging: general overview. Korean J Radiol. 2017; 18:570–584.

2. Obermeyer Z. Interview with Dr. Ziad Obermeyer on how collaboration between doctors and computers will help improve medical care. Available at:. http://www.nejm.org/action/showMediaPlayer?doi=10.1056%2FNEJMp1705348&aid=NEJMp1705348_attach_1&area=. Published 2017. Accessed Apr 20,. 2018.
3. The Lancet. Artificial intelligence in health care: within touching distance. Lancet. 2017; 390:2739.
5. No authors listed. AI diagnostics need attention. Nature. 2018; 555:285.
6. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanas-wamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016; 316:2402–2410.

7. Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017; 318:2211–2223.

8. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017; 318:2199–2210.

9. Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate classification of diminutive colorectal polyps using computer-aided analysis. Gastroenterology. 2018; 154:568–575.

10. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017; 284:574–582.

11. Larson DB, Chen MC, Lungren MP, Halabi SS, Stence NV, Langlotz CP. Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology. 2018; 287:313–322.

12. Yasaka K, Akai H, Abe O, Kiryu S. Deep learning with convolutional neural network for differentiation of liver masses at dynamic contrast-enhanced CT: a preliminary study. Radiology. 2018; 286:887–896.
13. Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S. Liver fibrosis: deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images. Radiology. 2018; 287:146–155.

14. Clarifai, Inc. Available at:. https://www.clarifai.com/tech-nology. Accessed Apr 18,. 2018.
15. Park SH, Han K. Methodologic guide for evaluating clinical performance and effect of artificial intelligence technology for medical diagnosis and prediction. Radiology. 2018; 286:800–809.
Go to : 
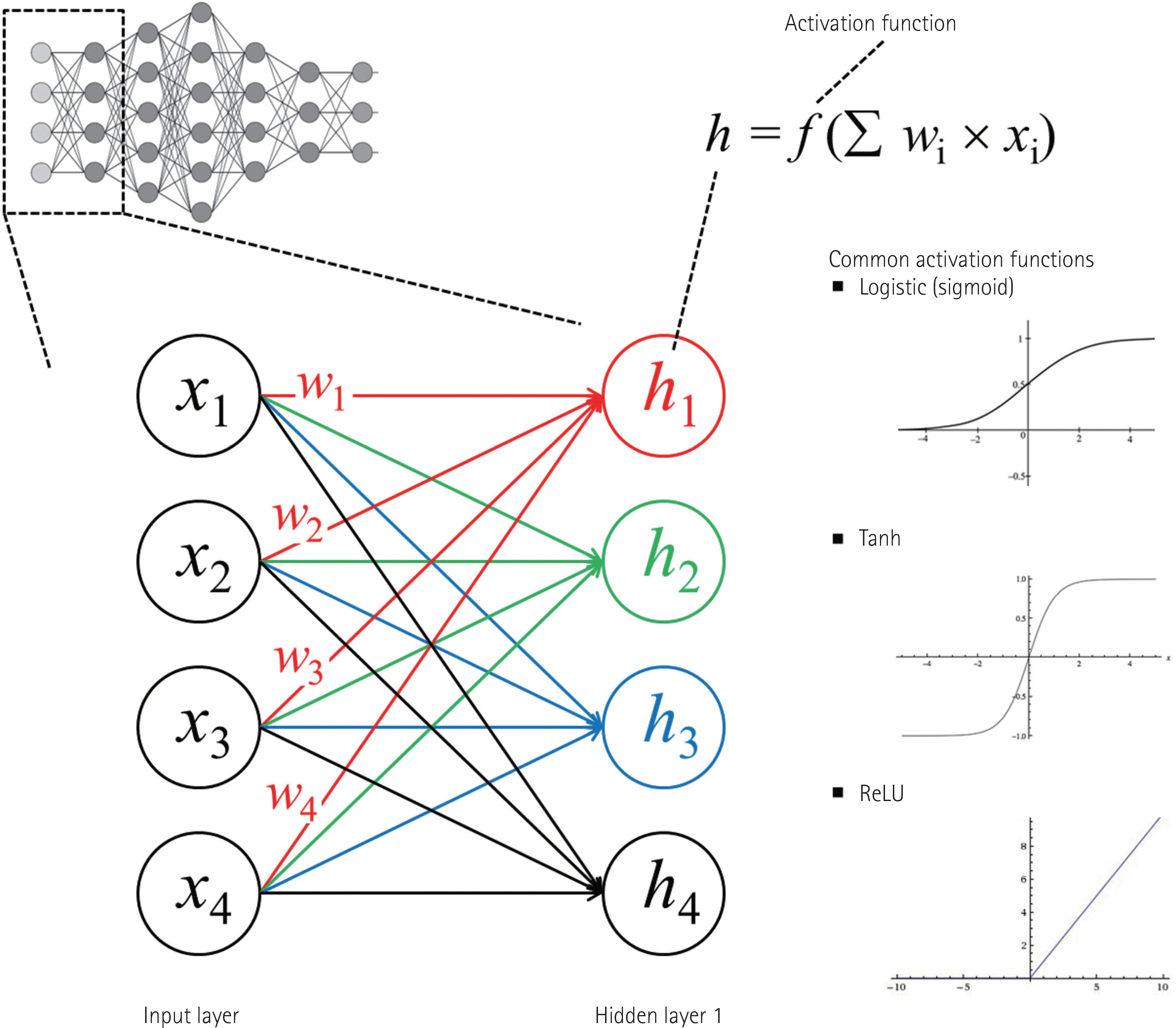 | Fig. 1.A diagram of artificial neural network consisting of multilayer perceptron. This simple diagram is for a conceptual explanation. When the logistic function is used as the activation function, the connection between all nodes (all x variables) in the input layer and one each node in hidden layer 1 makes a separate logistic function. Therefore, four different logistic functions (h1 to h4) marked by different colors (red, green, blue, and black) are created to connect the input layer to hidden layer 1 in this example. Other functions such as the tanh or the ReLU can be used as an activation function. Please see the main text for further explanations. ReLU = rectified linear unit, Tanh = hyperbolic tangent |
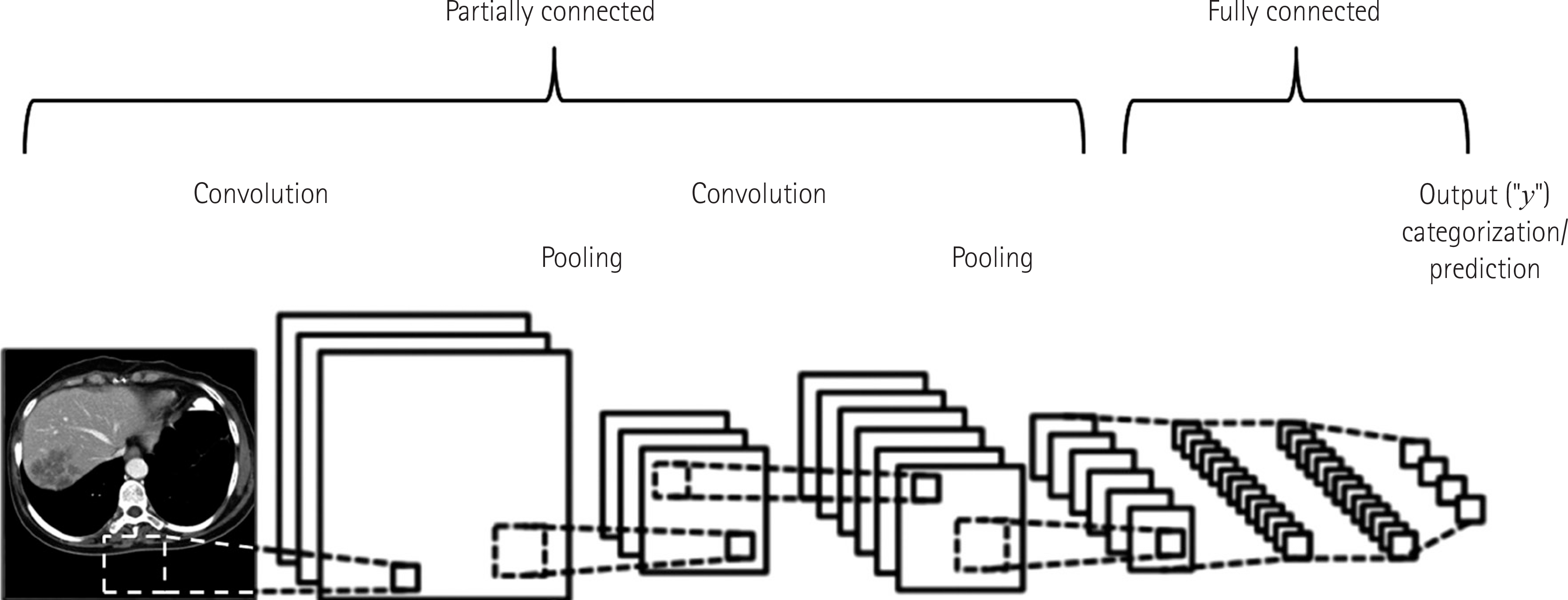 | Fig. 2.A diagram of convolutional neural network. This simple diagram is for a conceptual explanation. A typical convolutional neural network algorithm contains a much greater number of convolution and pooling steps and layers. Adapted from a background image available on the Internet (14). |
Table 1.
Opinions on Artificial Intelligence in Medicine Recently Published in Premier Medical and Scientific Journals




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download