Abstract
Pulmonary metastases present a wide spectrum of radiological findings, some of which have been known to be useful for analogizing the possible origin or site of primary tumors. In the present report, we describe a unique case of pulmonary metastasis manifesting on chest computed tomography as multiple nodules with tortuous, serpentine, aneurysmal, dilated, inner intratumoral vessels. The metastasis originated from uterine sarcoma.
Pulmonary metastases are common and can be classified according to the growth site, number of tumors, and pattern of radiological manifestations. With respect to the seeding mechanism, pulmonary metastases usually develop when tumor cells lodge in a small distal arteriole or in the interstitium. However, sometimes the cancerous cells are deposited as an endobronchial lesion (1). Pulmonary metastases most commonly present as multiple nodules or masses; however, in some cases of colorectal cancer or skeletal sarcoma, solitary metastases are predominant. Furthermore, pulmonary metastases can manifest in various patterns, including “military pattern,” “consolidation with air bronchogram,” “cavity,” and “perinodular” or “peritumoral ground-glass opacity” (2). These radiological features are helpful in determining the primary site of malignancy. However, to the best of our knowledge, only a few reports have documented chest computed tomography (CT) showing a dilated vascular structure in metastatic nodules or masses. Herein, we present a unique case of pulmonary metastasis manifesting as multiple nodules with tortuous, serpentine, aneurysmal, dilated, intratumoral vessels, which originated from uterine sarcoma.
A 60-year-old woman presented with generalized weakness, dyspnea, and weight loss that had persisted for 5 months. Her medical history revealed hypertension diagnosed two years earlier. After admission, she underwent all available routine laboratory tests, which displayed the following abnormal laboratory findings: increased white blood cell count (17400/µL) and high level of C-reactive protein (23.3 mg/dL), suggesting active inflammation throughout the body. Moreover, her hemoglobin level was 5.3 g/dL, mean corpuscular volume was 70.9 fL, serum iron level was 22 µg/dL, and ferritin level was 1478 ng/mL, suggesting microcytic anemia with iron deficiency.
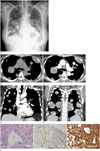
She underwent radiological testing, and a chest radiograph showed diffuse, variably sized, nodular opacities in both lungs (Fig. 1A). They were well-defined round masses. For further evaluation, she underwent chest CT. Pre-contrast axial chest CT showed multiple, variably sized, well-defined, round-to-ovoid soft tissue nodules and masses without any inner calcific foci in both lungs (Fig. 1B). Contrast-enhanced CT revealed multiple diffuse, heterogeneous, enhancing nodules and masses with tortuous, serpentine, or engorged inner vascular structures (Fig. 1C-E). We first considered the possibility of pulmonary vascular pseudoaneurysms associated with pulmonary vasculitis or septic pneumonia. However, the vascular structures did not have any direct connection with the pulmonary artery or its branches. They were confined within the masses and nodules. Some nodules had a low attenuation portion, suggesting internal necrosis. Collectively, these findings suggested pulmonary metastasis. Laboratory examination revealed that the patient's serum cancer antigen-125 level was markedly elevated (245.2 U/mL; normal: < 35 U/mL). Subsequently, for further evaluation, she underwent pelvic ultrasonography, which revealed a 6.6-cm, heterogeneous, irregular, putative primary mass in the uterus. She then underwent abdominal CT to rule out other malignancies, such as ovarian cancer. The abdominal CT showed a large, cystic, uterine mass with a partially enhancing solid portion in the right uterine wall; the mass was regarded as uterine malignancy, and a biopsy was recommended, but the patient refused the procedure. Therefore, a percutaneous core-needle biopsy of a nodule in the right lower lobe of the lung was performed. Microscopic examination revealed multiple cystic spaces with a flat, endothelial-like cell lining, indicating vascular spaces, and the tumors proved to be lung metastases derived from sarcomatous carcinoma (Fig. 1F). The patient underwent one course of palliative chemotherapy, but her general condition worsened. After discussions with her family, she decided not to undergo any more aggressive treatments, such as chemotherapy and radiotherapy. She was then transferred to another hospital in her hometown for conservative treatment.
In this report, we have presented a unique case of multiple, variably sized, pulmonary metastatic masses and nodules showing engorged, aneurysmal, and dilated tumor vessels, and these metastatic masses and nodules originated from uterine sarcoma.
Pulmonary metastases are common and show a wide range of radiological features. On one hand, these features help in determining the original tumor site; on the other hand, as in the present study, the unusual radiological features of some metastases make it difficult to distinguish them from non-malignant pulmonary diseases (2). The various known features of lung metastases are cavitation, calcification, hemorrhage around the metastatic nodules, pneumothorax, air-space pattern, tumor embolism, endobronchial metastasis, solitary mass, and dilated vessels within a mass (2). Squamous cell carcinoma is the most common cancer type that yields a cavitating metastasis with a thick and irregular wall. In contrast, thin-walled cavities can be observed in metastases from sarcomas and adenocarcinomas. Furthermore, calcification can occur in metastatic sarcomas or adenocarcinomas, making it difficult to differentiate these diseases from benign granulomas or hamartomas. Peritumoral hemorrhage results in areas of nodular attenuation surrounded by a halo of ground-glass opacity. Pneumothorax commonly occurs as a result of metastases from an osteosarcoma. Airspace consolidation is often seen in metastases from gastrointestinal tract malignancies, whereas a common radiological manifestation of endobronchial metastasis is atelectasis (2). Therefore, these findings are helpful in the differential diagnosis of pulmonary masses, even though they are not specific.
Dilated vascular structures within the masses occur in cases of metastasis from a sarcoma, in particular, alveolar soft-part sarcoma or leiomyosarcoma (2). Some studies have shown lung metastases from soft-part sarcoma, which contain dilated intratumoral vessels; however, there are few cases of lung metastases from uterine sarcoma (34).
Uterine sarcomas are rare neoplasms, comprising 5% of uterine malignancies (5), and uterine leiomyosarcoma is the second most common subtype of uterine sarcoma, accounting for 30–40% of all uterine sarcomas (56). Leiomyosarcomas are the most malignant smooth muscle tumors of the uterus, and they show the microscopic constellation of hypercellularity, severe nuclear atypia, and high mitotic rate. Sahdev and colleagues reported that most uterine sarcomas show strong enhancement of the mass (7). The precise pathogenesis of engorged intratumoral vessels in tumors is unknown, but these engorged tumor vessels may be suggestive of the hypervascular nature of the metastatic nodules (8).
When a patient presents with dilated and tortuous vessels in lung masses, other vascular lesions should be ruled out, including arteriovenous fistulae, pulmonary artery aneurysms, and pulmonary vein varices. Furthermore, primary lung cancer and pulmonary metastases can cause erosion of the pulmonary arteries and result in pseudoaneurysm formation. In rare cases, primary tumors arising from the pulmonary arteries, such as leiomyosarcomas and angiosarcomas, can cause focal expansion and aneurysmal dilatation of the pulmonary artery itself (910). However, in the present case, the vascular structures did not show any direct connection with the pulmonary artery or its branches. Infection with tuberculosis bacteria, pyogenic bacteria, and fungi can also cause pseudoaneurysms (910). However, in such cases, the main findings are likely to be ground-glass opacity or consolidation, reflecting infection or inflammation of the lung, rather than multiple metastatic nodules.
In summary, we have presented a unique case of pulmonary metastasis manifesting as multiple nodules with well-developed, tortuous, serpentine, aneurysmal, dilated inner intratumoral vessels, and the metastasis may have originated from uterine sarcoma.
Figures and Tables
Fig. 1
A 60-year-old woman with pulmonary metastasis from uterine sarcoma, presenting as multiple nodules with tortous, serpentine, aneurysmal dilated intraumor vessels.
A. Initial chest radiograph shows multiple, round, variably sized nodules and masses in both lungs, as well as a small amount of pleural effusion.
B. Pre-enhanced image shows well-defined homogeneous, variably sized, soft-tissue nodules with a density similar to that of the back muscles and no inner calcific foci.
C. Axial contrast-enhanced CT image shows aneurysmal dilatated intratumoral vessel (thick arrow). It has no connection with adjacent branch of the pulmonary artery (thin arrow).
D. Coronal contrast-enhanced CT image also show aneurysmal dilatated intratumoral vessels (thick arrows). Branches of the pulmonary artery (thin arrows) pass through the nodules without any connection.
E. Coronal contrast-enhanced CT image show multiple variable sized nodues and masses with tortous, dilatated intratumoral or intranodal vessels with an inner cystic or necrotic portion.
F. Photomicrographs show tumor cell infiltration with circumscribed cystic spaces lined with flat, endothelial-like cells (arrow), suggesting vascular structures (hematoxylin and eosin, × 100). Cystic spaces show CD-31 positivity (CD-31, × 100) and tumor cells react strongly with mesenchymal antigen (Vimentin, × 400).
CD-31 = cluster of differentiation-31, CT = computed tomography
References
1. Davis SD. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology. 1991; 180:1–12.

2. Seo JB, Im JG, Goo JM, Chung MJ, Kim MY. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001; 21:403–417.

3. Daly BD, Cheung H, Gaines PA, Bradley MJ, Metreweli C. Imaging of alveolar soft part sarcoma. Clin Radiol. 1992; 46:253–256.

4. Choi JI, Goo JM, Seo JB, Kim HY, Park CK, Im JG. Pulmonary metastases of alveolar soft-part sarcoma: CT findings in three patients. Korean J Radiol. 2000; 1:56–59.

5. Shah SH, Jagannathan JP, Krajewski K, O'Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012; 199:213–223.

6. Tirumani SH, Deaver P, Shinagare AB, Tirumani H, Hornick JL, George S, et al. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J Gynecol Oncol. 2014; 25:306–312.

7. Sahdev A, Sohaib SA, Jacobs I, Shepherd JH, Oram DH, Reznek RH. MR imaging of uterine sarcomas. AJR Am J Roentgenol. 2001; 177:1307–1311.

8. Ueda M, Otsuka M, Hatakenaka M, Sakai S, Ono M, Yoshimitsu K, et al. MR imaging findings of uterine endometrial stromal sarcoma: differentiation from endometrial carcinoma. Eur Radiol. 2001; 11:28–33.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download