Abstract
Leukocytoclastic vasculitis is a rare disease characterized by neutrophil and immune-complex deposition in the small vessel walls. We report a 47-year-old female patient with leukocytoclastic vasculitis of the breast, presenting as breast edema on mammography, irregular hypoechoic lesion with central necrosis on ultrasonography and regionally distributed heterogeneous non-mass enhancement on magnetic resonance imaging.
Leukocytoclastic vasculitis is an immune complex-mediated small-vessel vasculitis and characterized by the inflammation of small vessels showing leukocytoclasis (destructive fragmentation of the nucleus of a dying cell) of infiltrating neutrophils, fibrinoid necrosis of the vascular wall and subsequent extravasation of erythrocytes (1). Here, we describe a case of the breast involvement of leukocytoclastic vasculitis and present the imaging findings of mammography, ultrasonography (US) and magnetic resonance imaging (MRI).
A 47-year-old woman visited our hospital for palpable purpura, skin ulceration and necrosis of her right breast. One month ago, erythematous skin change developed in her right breast, especially in the nipple and periareolar area. Before the symptom developed, she taped her nipples for two days when she worked during the daytime. She visited the local private clinic and took antibiotics for 3 weeks. However the skin ulceration and necrosis aggravated (Fig. 1A). She didn't have any medical history nor family history of breast cancer. On laboratory findings, the levels of erythrocyte sedimentation rate (73 mm/hr, normal range: 0–25 mm/hr) and C-reactive protein (1.51 mg/dL, normal range: 0.02–0.8 mg/dL) were elevated. Other routine blood counts and biochemistry were normal. Blood culture did not show any bacterial or fungal growth.
Mammography was performed using Lorad Selenia (Hologic Inc., Bedford, MA, USA). On mammography, there was focal asymmetry in the subareolar area with diffuse skin thickening and trabecular thickening of right breast (Fig. 1B). Breast US was performed using a HI VISION Ascendus (Hitachi-Aloka Medical Ltd., Tokyo, Japan) with a 5–18 MHz transducer. On US, there was an irregular hypoechoic lesion with central necrosis at right subareolar area (Fig. 1C). There was associated skin thickening and soft tissue edema of right breast. Reactive lymph node hyperplasia was noted in right axilla. Breast MRI was performed using a 1.5-T system (Signa HDxt, GE Healthcare, Milwaukee, WI, USA) with a dedicated eight-channel breast coil. On contrast enhanced fat-suppressed T1-weighted image, there was regional distributed heterogeneous non-mass enhancement in right central breast involving the right nipple and periareolar area (Fig. 1D). On kinetic curve assessment, it showed initial fast and delayed washout pattern. Given the clinical and imaging findings, our impressions were infectious mastitis or idiopathic granulomatous mastitis. US-guided core needle biopsy was done and the pathologic result was acute and chronic inflammation.
She underwent the debridement of skin and soft tissue. On gross examination, cut surface of the specimen showed ulceration and discoloration of the skin with diffusely necrotic dermis. Microscopic examination (Fig. 1E) revealed leukocytoclastic vasculitis characterized by small vessel damage by nuclear debris from infiltrating neutrophils. Massive intravascular fibrin thrombi were also noted at superficial vessels. After the pathologic results, for the differentiation of autoimmune vasculitis, additional laboratory tests were performed. Anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, complement C3 and C4, rheumatoid factor, cryoglobulin and parasite IgG were negative.
The patient began steroid treatment and her symptoms resolved. There were no abnormal findings in her breasts on followup US 12 months later.
Leukocytoclastic vasculitis, also known as hypersensitivity vasculitis is an immune complex-mediated small-vessel vasculitis causing 10–20% of all small-vessel vasculitis (2). Leukocytoclastic vasculitis is characterized by the inflammation of small vessels, mainly post-capillary venules showing leukocytoclasis (destructive fragmentation of the nucleus of a dying cell) of infiltrating neutrophils, fibrinoid necrosis of the vascular wall and subsequent extravasation of erythrocytes (1). The most common symptom of leukocytoclastic vasculitis is palpable purpura. Other less common symptoms are urticarial plaques, vesicles, bullae and pustules. For the diagnosis of leukocytoclastic vasculitis, other causes such as infection, connective tissue disease or malignancy should be ruled out by clinical laboratory finding or tissue biopsy.
Systemic vasculitis denotes the inflammation of blood vessels involving multiple organs and when the vasculitis is restricted to single organ, the term single organ vasculitis is used (3). Single organ vasculitis may affect small, medium or large sized vessels and the patterns of inflammation may be granulomatous or non-granulomatous. Long-term follow-up more than 6 months is needed, because single organ vasculitis may evolve into a systemic vasculitis in rare cases (3). There have been several studies reporting the giant cell arteritis, polyarteritis nodosa and Wegener's granulomatosis involving the breast (45). A complex cystic and solid mass may be observed on US in patient of polyarteritis nodosa involving the breast (5). Irregular mass could be observed in patient with Wegener's granulomatosis on mammography and US mimicking breast malignancy (6). Lee and Joo (7) reported the imaging finding of isolated breast vasculitis. It was manifested as bilateral breast edema on mammography and there was hypoechoic circumferential arterial wall thickening with perivascular fat infiltrations on US.
To the best of our knowledge, the imaging finding of leukocytoclastic vasculitis involving the breast has not been reported. In our case, when the erythematous skin change began, she had taped her nipples for two days when she worked during the daytime. We supposed that some toxic material of the tape could cause hypersensitivity reactions in this patient. In our case, there was edema of the affected showing diffuse skin thickening and trabecular thickening on mammography. On US, there was an irregular hypoechoic lesion with central necrosis at subareolar area with associated skin thickening and increased echogenicity of subcutaneous fat. On MRI, this lesion was presented as regional distributed heterogeneous non-mass enhancement. Given the imaging findings of three imaging modalities, the most important differential diagnosis could be the idiopathic granulomatous mastitis. Mammographic features of idiopathic granulomatous mastitis include focal asymmetries, masses, and skin thickening and US features are hypoechoic masses, heterogeneous parenchyma and abscess (8). Non-mass enhancement is the most common finding of idiopathic granulomatous mastitis followed by the architectural distortion and skin thickening (9). Thus, mammographic, US and MRI findings of idiopathic granulomatous mastitis are very similar to our case and it is difficult to differentiate these two diseases based on imaging findings. However, our patient accompanied severe skin changes such as erythema, palpable purpura, ulceration and necrosis and these clinical symptoms favor the diagnosis of leukocytoclastic vasculitis of the breast.
Treatment of leukocytoclastic vasculitis often does not require aggressive therapy due to a usually favorable course, and corticosteroids are indicated when there are signs of skin necrosis (10).
In conclusion, we report a case of breast involvement by leukocytoclastic vasculitis. If there is breast abscess and edema accompanied by skin changes such as palpable purpura or ulceration, the possibility of leukocytoclastic breast should be considered. Despite its rarity, if radiologists recognize clinical and radiographic findings, the possibility of leukocytoclastic vasculitis should be considered as a differential diagnosis. It could be helpful in making appropriate treatment decision.
Figures and Tables
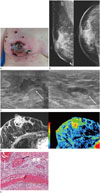 | Fig. 1A 47-year-old woman with leukocytoclastic vasculitis of right breast.
A. Patient showing erythematous skin changes, ulceration and necrosis of right breast.
B. Mammographic images show focal asymmetry in the subareolar area with diffuse skin thickening and trabecular thickening of right breast.
C. Ultrasound shows the presence of an irregular hypoechoic lesion with central necrosis (an arrow in the left image) in the right subareolar area. Reactive lymph node hyperplasia (an arrow in the right image) is noted in right axilla.
D. The contrast-enhanced fat-suppressed T1-weighted image reveals regionally distributed heterogeneous non-mass enhancement in the right central breast with involvement of the right nipple and periareolar area (left). Color map image obtained with computer-aided diagnosis (right), shows the lesion containing a central red portion suggesting initial rapid and delayed washout pattern of kinetic analysis.
E. Histological examination (hematoxylin and eosin stain, × 100) reveals several infiltrating neutrophils in the small vessel wall causing vessel wall damage (black arrow). Massive intravascular fibrin thrombi are also noted in the superficial vessels (white arrow).
|
References
2. Dubost JJ, Souteyrand P, Sauvezie B. Drug-induced vasculitides. Baillieres Clin Rheumatol. 1991; 5:119–138.

3. Hernández-Rodríguez J, Hoffman GS. Updating single-organ vasculitis. Curr Opin Rheumatol. 2012; 24:38–45.

4. Kadotani Y, Enoki Y, Itoi N, Kojima F, Kato G, Lee CJ. Giant cell arteritis of the breast: a case report with a review of literatures. Breast Cancer. 2010; 17:225–232.

5. Khalil HH, Marsden J, Akbar N, Gordon P, Roberts J, Schulte KM. Polyarteritis nodosa of the breast: presentation and management. Int J Surg. 2009; 7:446–450.

6. Szabo-Moskal J. Wegener's granulomatosis of the breast: a case report. Pol J Radiol. 2014; 79:117–119.

7. Lee JY, Joo M. Isolated breast vasculitis manifested as breast edema with suggestive sonographic findings: a case report with imaging findings. J Med Ultrason. 2017; 44:191–195.

8. Ozturk M, Mavili E, Kahriman G, Akcan AC, Ozturk F. Granulomatous mastitis: radiological findings. Acta Radiol. 2007; 48:150–155.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download