Abstract
Purpose
To assess the usefulness of an ultrasound (US)-guided peritoneal biopsy for the solitary peritoneal thickening visualized as only infiltrated fat on a computed tomography (CT) scan.
Materials and Methods
This retrospective study included 36 patients (16 males, 20 females; mean age, 51.7 years) who underwent a US-guided biopsy for the solitary peritoneal thickening of unknown cause visualized as only infiltrated fat without an apparent mass formation on a CT scan. The rate of the specific histopathological diagnosis and accuracy for the diagnosis of malignant disease was assessed.
Results
The procedure was technically successful with the acquisition of an adequate amount of the specimen for microscopic examination from all patients. A specific histopathological diagnosis was made in 31/36 patients (86.1%): peritoneal carcinomatosis in 15/31 (48.4%), tuberculous peritonitis in 15/31 (48.4%) and panniculitis in 1/31 (3.2%). A non-specific histopathological diagnosis was made in 5/36 (13.9%): chronic inflammation in 4/5 (80%) and mesothelial hyperplasia in 1/5 (20%). The procedure showed sensitivity of 83.3%, with a specificity of 100%, a positive predictive value of 100%, a negative predictive value of 85.7%, and an accuracy rate of 86.1% for the diagnosis of malignant diseases.
Go to : 
References
1. Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008; 28:583–607.
2. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009; 29:347–373.

3. Kang SJ, Kim JW, Baek JH, Kim SH, Kim BG, Lee KL, et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J Gastroenterol. 2012; 18:2837–2843.

4. Xiao WB, Liu YL. Elevation of serum and ascites cancer antigen 125 levels in patients with liver cirrhosis. J Gastroenterol Hepatol. 2003; 18:1315–1316.

5. Simsek H, Savas MC, Kadayifci A, Tatar G. Elevated serum CA 125 concentration in patients with tuberculous peritonitis: a case-control study. Am J Gastroenterol. 1997; 92:1174–1176.
6. Hiller N, Lioubashevsky N. Tuberculous peritonitis: a diagnostic challenge. Abdom Imaging. 2001; 26:319–322.

7. Filippone A, Cianci R, Delli Pizzi A, Esposito G, Pulsone P, Tavoletta A, et al. CT findings in acute peritonitis: a pattern-based approach. Diagn Interv Radiol. 2015; 21:435–440.

8. Charoensak A, Nantavithya P, Apisarnthanarak P. Abdominal CT findings to distinguish between tuberculous peritonitis and peritoneal carcinomatosis. J Med Assoc Thai. 2012; 95:1449–1456.
9. Vázquez Muñoz E, Gómez-Cerezo J, Atienza Saura M, Vázquez Rodriguez JJ. Computed tomography findings of peritoneal tuberculosis: systematic review of seven patients diagnosed in 6 years (1996–2001). Clin Imaging. 2004; 28:340–343.
10. Chen R, Chen Y, Liu L, Zhou X, Liu J, Huang G. The role of 18F-FDG PET/CT in the evaluation of peritoneal thickening of undetermined origin. Medicine (Baltimore). 2016; 95:e3023.

11. Wang SB, Ji YH, Wu HB, Wang QS, Zhou WL, Lv L, et al. PET/CT for differentiating between tuberculous peritonitis and peritoneal carcinomatosis: the parietal peritoneum. Medicine (Baltimore). 2017; 96:e5867.
12. Suzuki A, Kawano T, Takahashi N, Lee J, Nakagami Y, Miyagi E, et al. Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging. 2004; 31:1413–1420.

13. Târcoveanu E, Dimofte G, Bradea C, Lupas¸cu C, Moldovanu R, Vasilescu A. Peritoneal tuberculosis in laparoscopic era. Acta Chir Belg. 2009; 109:65–70.

14. Bedioui H, Ksantini R, Nouira K, Mekni A, Daghfous A, Chebbi F, et al. Role of laparoscopic surgery in the etiologic diagnosis of exsudative ascites: a prospective study of 90 cases. Gastroenterol Clin Biol. 2007; 31:1146–1149.

15. Abdelaal A, Alfkey R, Abdelaziem S, Abunada M, Alfaky A, Ibrahim WH, et al. Role of laparoscopic peritoneal biopsy in the diagnosis of peritoneal tuberculosis. A seven-year experience. Chirurgia (Bucur). 2014; 109:330–334.
16. Hong KD, Lee SI, Moon HY. Comparison between laparoscopy and noninvasive tests for the diagnosis of tuberculous peritonitis. World J Surg. 2011; 35:2369–2375.

17. Perugini RA, Callery MP. Complications of laparoscopic surgery. In Holzheimer RG, Mannick JA, eds. Surgical treatment: evidencebased and problem-oriented. Munich: Zuckschw-erdt. 2001.
18. Beleña JM, Nuñez M. Postoperative complications of laparoscopic surgery. Int J Clin Anesthesiol. 2014; 2:1034.
19. Souza FF, Mortelé KJ, Cibas ES, Erturk SM, Silverman SG. Predictive value of percutaneous imaging-guided biopsy of peritoneal and omental masses: results in 111 patients. AJR Am J Roentgenol. 2009; 192:131–136.

20. Wang J, Gao L, Tang S, Li T, Lei Y, Xie H, et al. A retrospective analysis on the diagnostic value of ultrasound-guided percutaneous biopsy for peritoneal lesions. World J Surg Oncol. 2013; 11:251.

21. Que Y, Wang X, Liu Y, Li P, Ou G, Zhao W. Ultrasound-guided biopsy of greater omentum: an effective method to trace the origin of unclear ascites. Eur J Radiol. 2009; 70:331–335.

22. Mahmood K, Saeedi MI, Mohammad R, Ziauddin , Kamal M. Percutaneous needle peritoneal biopsy in the diagnosis of exudative ascites. J Ayub Med Coll Abbottabad. 2008; 20:94–96.
23. Khati NJ, Gorodenker J, Hill MC. Ultrasound-guided biopsies of the abdomen. Ultrasound Q. 2011; 27:255–268.

24. Kim YH, Ryeom HK, Chung TG, Park HY, Kim YJ, Kang DS. Ultrasound-guided biopsy of the thickened peritoneal reflections: efficacy and diagnostic role in the differential diagnosis of peritoneal tuberculosis and peritoneal carcinomatosis. J Korean Radiol Soc. 2000; 43:215–221.

25. Mehta JB, Dutt A, Harvill L, Mathews KM. Epidemiology of extrapulmonary tuberculosis: a comparative analysis with pre-AIDS era. Chest. 1991; 99:1134–1138.
26. Cegielski JP, Chin DP, Espinal MA, Frieden TR, Rodriquez Cruz R, Talbot EA, et al. The global tuberculosis situation: progress and problems in the 20th century, prospects for the 21st century. Infect Dis Clin North Am. 2002; 16:1–58.

27. Gore RM, Miller FH, Yaghmai V. Acquired immunodeficiency syndrome (AIDS) of the abdominal organs: imaging features. Semin Ultrasound CT MR. 1998; 19:175–189.

29. Ji HL, Nie HG. Electrolyte and fluid transport in mesothelial cells. J Epithel Biol Pharmacol. 2008; 1:1–7.

30. Smith EH. Complications of percutaneous abdominal fine-needle biopsy review. Radiology. 1991; 178:253–258.

Go to : 
 | Fig. 1.A 59-year-old man with peritoneal carcinomatosis with unknown primary site. A. CT of the abdomen shows mild thickening of the greater omentum (arrows) with moderate amount of ascites. B. Transverse ultrasonogram (5 MHz convex-array transducer) shows minimally thickened greater omentum (arrows) and small amount of ascites. On color Doppler examination, no prominent vessels can be found. Note the bowel loops (asterisks) posterior aspect of thickened greater omentum. C. Transverse ultrasonogram obtained during the biopsy reveals the thickened omentum (arrows) with well-visualized and well-placed biopsy needle (arrowheads). Note the adjacent bowel loops (asterisks) near the biopsy needle and ascites around the targeted omentum. The histopathological diagnosis by ultrasound-guided biopsy was the peritoneal carcinomatosis. D. Follow-up CT of the abdomen 9months after chemotherapy shows aggravated peritoneal thickening which is ‘omental cake' formation (arrows). CT = computed tomography |
 | Fig. 2.A 17-year-old woman with tuberculous peritonitis. A. CT of the abdomen shows small amount of ascites and mild thickening of the greater omentum with only fatty infiltration (asterisks) and parietal peritoneum (arrows) with contrast enhancement. B. Transverse ultrasonogram (12 MHz linear-array transducer) shows thickened greater omentum with 2.08 cm thickness (arrows). C. Transverse ultrasonogram during the biopsy shows well placed biopsy needle (arrowheads) which is slightly angulated pathway to obtain as much sample as possible. The histopathological diagnosis by ultrasound-guided biopsy was the tuberculous peritonitis. D. Follow-up CT of the abdomen shows resolution of peritoneal thickening and ascites after anti-tuberculosis medication. CT = computed tomography |
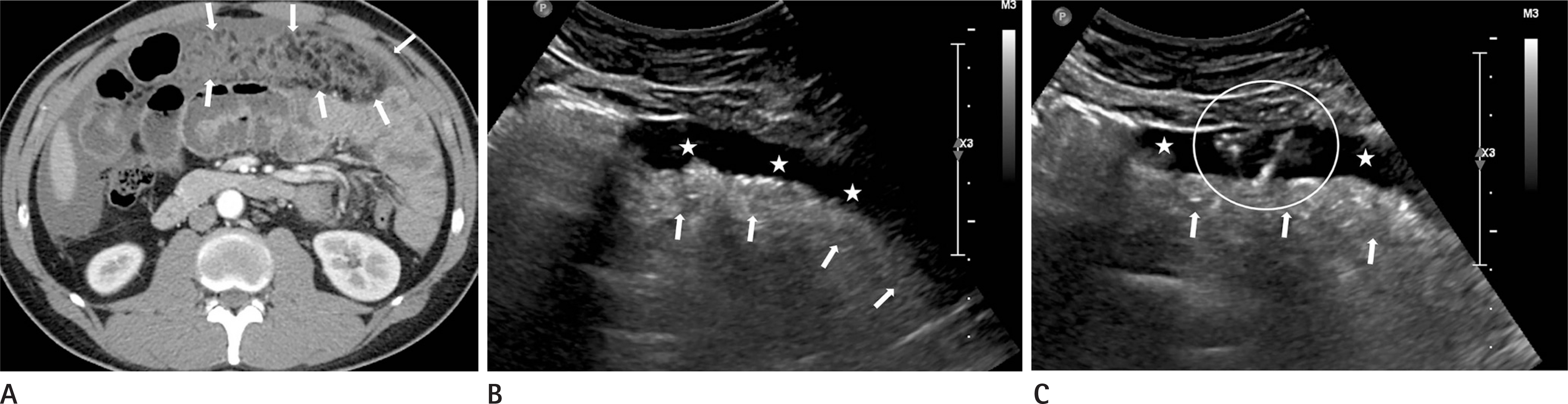 | Fig. 3.A 39-year-old man with peritoneal carcinomatosis. A. CT scan of the abdomen shows thickening of the greater omentum with fatty infiltration (arrows) and small amount of ascites. B, C. Transverse ultrasonogram (5 MHz convex-array transducer) shows thickened greater omentum (arrows) that corresponded to the lesion selected as biopsy site on CT. There is ascites (asterisks) around echogenic omentum (arrows). Transverse ultrasonogram obtained immediately after the biopsy shows tiny moving echogenic dots emerged from the biopsy site (echogenic dots and tubules within the circle), which represent active bleeding. This bleeding was stopped within 5 minutes without specific management. CT = computed tomography |
Table 1.
Underlying Disease, Histopathological Diagnosis by US-Guided Peritoneal Biopsy and Finalized Diagnosis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download