Abstract
Purpose
The purpose of this study was to investigate the immune-histochemical characteristics of upgraded malignancy from high-risk and borderline breast lesions, and to correlate the upgrade rates with clinical findings.
Materials and Methods
We scrutinized image-guided biopsy records retrospectively, and included all women afflicted with high-risk and borderline breast lesions during the period, 2011 to 2015, inclusive. A total of 340 high-risk and borderline lesions were identified by the pathologist in biopsy samples and thereafter, surgical excision and/or image follow-up for at least 24 months was performed. We compared the clinical emanating from both high-risk and borderline lesions, and with and without cancer upgrade. In the instances of lesions with cancer upgrade, histologic and immuohistochemical reviews were performed.
Results
Of the 340 high-risk or borderline lesions, 18.8% (64/340) were upgraded. The upgrade rates were higher in patients of more advanced age, larger body habitus and afflicted with atypical ductal hyperplasia rather than with other pathology (p < 0.05). In the lesions with cancer upgrade (n = 64), there was no lymph node metastasis. The estrogen receptor-positive (93.8%), progesterone receptor-positive (87.5%), human epidermal growth factor receptor type 2-negative (90.6%), Ki-67-negative (82.8%), and Luminal A (76.6%) types were seen more frequently.
References
1. Londero V, Zuiani C, Linda A, Battigelli L, Brondani G, Bazzocchi M. Borderline breast lesions: comparison of malignancy underestimation rates with 14-gauge core needle biopsy versus 11-gauge vacuum-assisted device. Eur Radiol. 2011; 21:1200–1206.

2. Londero V, Zuiani C, Linda A, Girometti R, Bazzocchi M, Sardanelli F. High-risk breast lesions at imaging-guided needle biopsy: usefulness of MRI for treatment decision. AJR Am J Roentgenol. 2012; 199:W240–W250.

3. Menes TS, Rosenberg R, Balch S, Jaffer S, Kerlikowske K, Miglioretti DL. Upgrade of high-risk breast lesions detected on mammography in the Breast Cancer Surveillance Consortium. Am J Surg. 2014; 207:24–31.

4. Krishnamurthy S, Bevers T, Kuerer H, Yang WT. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol. 2012; 198:W132–W140.

5. Sewell CW. Pathology of high-risk breast lesions and ductal carcinoma in situ. Radiol Clin North Am. 2004; 42:821–830.

6. Linda A, Zuiani C, Bazzocchi M, Furlan A, Londero V. Borderline breast lesions diagnosed at core needle biopsy: can magnetic resonance mammography rule out associated malignancy? Preliminary results based on 79 surgically excised lesions. Breast. 2008; 17:125–131.

7. Heller SL, Moy L. Imaging features and management of highrisk lesions on contrast-enhanced dynamic breast MRI. AJR Am J Roentgenol. 2012; 198:249–255.

8. Pediconi F, Padula S, Dominelli V, Luciani M, Telesca M, Casali V, et al. Role of breast MR imaging for predicting malignancy of histologically borderline lesions diagnosed at core needle biopsy: prospective evaluation. Radiology. 2010; 257:653–661.

9. Kohr JR, Eby PR, Allison KH, DeMartini WB, Gutierrez RL, Peacock S, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology. 2010; 255:723–730.

10. Lips EH, Mulder L, de Ronde JJ, Mandjes IA, Koolen BB, Wessels LF, et al. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat. 2013; 140:63–71.

11. Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA. Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol. 2010; 20:1111–1117.

12. Bae MS, Park SY, Song SE, Kim WH, Lee SH, Han W, et al. Heterogeneity of triple-negative breast cancer: mammographic, US, and MR imaging features according to androgen receptor expression. Eur Radiol. 2015; 25:419–427.

13. Kim SH, Seo BK, Lee J, Kim SJ, Cho KR, Lee KY, et al. Correlation of ultrasound findings with histology, tumor grade, and biological markers in breast cancer. Acta Oncol. 2008; 47:1531–1538.

14. Philpotts LE, Shaheen NA, Jain KS, Carter D, Lee CH. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000; 216:831–837.

15. Esserman L, Yau C. Rethinking the standard for ductal carcinoma in situ treatment. JAMA Oncol. 2015; 1:881–883.

16. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015; 1:888–896.

17. Carels N, Spinassé LB, Tilli TM, Tuszynski JA. Toward precision medicine of breast cancer. Theor Biol Med Model. 2016; 13:7.

18. Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008; 32:513–523.

19. Sinn HP, Elsawaf Z, Helmchen B, Aulmann S. Early breast cancer precursor lesions: lessons learned from molecular and clinical studies. Breast Care (Basel). 2010; 5:218–226.

20. Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of longterm follow-up. Cancer. 2005; 103:2481–2484.

Fig. 1.
Imaging findings of a 36-year-old woman who had a papillary neoplasm by US-guided core needle biopsy that was not surgically confirmed. A. On the US, there was a 0.6-cm-sized oval hypoechoic mass with microlobulated margins at 9 o'clock in the right breast, which was thought to be Breast Imaging Reporting and Data System category 4A (arrow). B. On the follow-up US after 5 years, the lesion had not changed (arrow). US = ultrasound
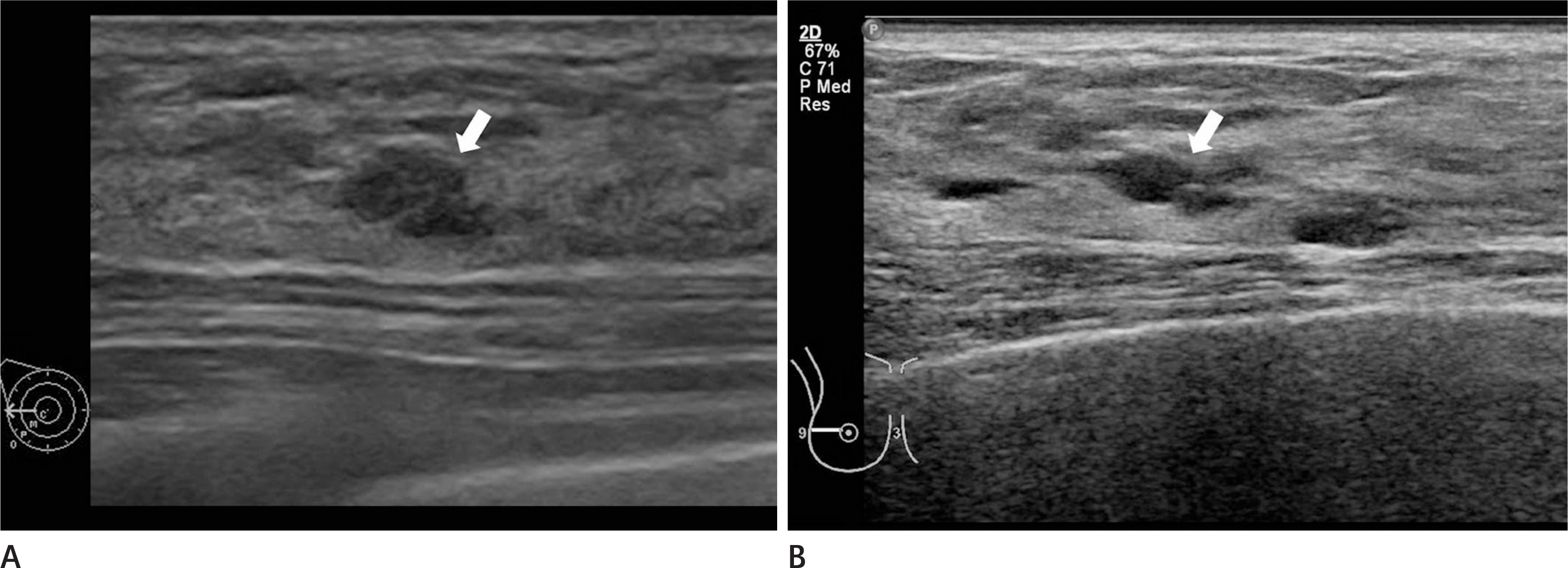
Fig. 2.
Imaging findings of a 56-year-old woman who had an ADH by US-guided core needle biopsy; it was upgraded to invasive ductal carcinoma after surgical excision. The immunohistochemical characteristics of this case were Luminal A [estrogen receptor (+), progesterone receptor (+), human epidermal growth factor receptor type 2 (−), Ki-67 (low), epidermal growth factor receptor (−)]. A. Right magnification view shows two areas of grouped amorphous microcalcifications in the middle and inner upper portions of the right breast, which were thought to be Breast Imaging Reporting and Data System category 4B (arrows). B, C. By both automated (B) and hand-held ultrasound (C), there was an irregular isoechoic mass with suspicious calcifications at 12 o'clock in the right breast, which correlated with a prior mammography (arrows). Through US-guided core needle biopsy, the lesion was confirmed as ADH. D. On the maximal intensity projection image of breast magnetic resonance imaging, there was a regional nonmass enhancement 2.6 × 1.5 cm in size at 12 o'clock in the right breast. E. The patient underwent mammography-guided localization and surgical excision. The specimen contained microcalcifications approximately 4 cm in extent (arrow) and was confirmed as invasive ductal carcinoma. ADH = atypical ductal hyperplasia, US = ultrasound

Table 1.
Clinical Characteristics and Pathology Results of High Risk and Borderline Lesions
Continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as number (percentage). ∗Between the ‘without cancer upgrade' and ‘with cancer upgrade' groups. ADH = atypical ductal hyperplasia, DCIS = ductal carcinoma in situ, Mammo = mammography, MSVAB = mammography-guided stereotactic vacuum-assisted biopsy, UCNB = ultrasound-guided core needle biopsy, USG = ultrasonography
Table 2.
Univariable and Multivariate Model of High Risk and Borderline Lesions to Upgrade Breast Cancer
Table 3.
Histopathologic and Molecular Characteristics of Upgraded Cancers from High-Risk and Borderline Lesions
| Initial Biopsy Result, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| ADH (n = 31)∗ | Lobular (n = 2) | Radial (n = 3) | Papillary (n = 21) | Flat (n = 4) | Mucocele (n = 3) | Total (n = 64)∗ | |
| Type of cancer | |||||||
| DCIS | 24 (77.4) | 0 (0) | 1 (33.3) | 16 (76.2) | 4 (100) | 1 (33.3) | 46 (71.9) |
| Invasive cancer | 7 (22.6) | 2 (100) | 2 (66.7) | 5 (23.8) | 0 (0) | 2 (66.7) | 18 (28.1) |
| DCIS grade | |||||||
| Low | 9 (37.5) | 0 (0) | 1 (100) | 7 (43.8) | 3 (75) | 0 (0) | 20 (43.5) |
| Intermediate | 12 (50) | 0 (0) | 0 (0) | 8 (50) | 0 (0) | 1 (100) | 21 (45.7) |
| High | 3 (12.5) | 0 (0) | 0 (0) | 1 (6.3) | 1 (25) | 0 (0) | 5 (10.9) |
| Invasive cancer grade | |||||||
| Well differentiated | 2 (28.6) | 1 (50) | 0 (0) | 1 (20) | 0 (0) | 2 (100) | 6 (33.3) |
| Moderately differentiated | 5 (71.4) | 1 (50) | 2 (100) | 4 (80) | 0 (0) | 0 (0) | 12 (66.7) |
| Poorly differentiated | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ER | |||||||
| Negative | 1 (3.2) | 0 (0) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 2 (3.1) |
| Positive | 28 (90.3) | 2 (100) | 3 (100) | 20 (95.2) | 4 (100) | 3 (100) | 60 (93.8) |
| PR | |||||||
| Negative | 1 (3.2) | 0 (0) | 1 (33.3) | 4 (19) | 0 (0) | 0 (0) | 6 (9.4) |
| Positive | 28 (90.3) | 2 (100) | 2 (66.7) | 17 (81) | 4 (100) | 3 (100) | 56 (87.5) |
| HER2 | |||||||
| Negative | 29 (93.5) | 2 (100) | 2 (66.7) | 18 (85.7) | 4 (100) | 3 (100) | 58 (90.6) |
| Positive | 0 (0) | 0 (0) | 1 (33.3) | 3 (14.3) | 0 (0) | 0 (0) | 4 (6.3) |
| Ki-67 | |||||||
| Negative | 22 (71.0) | 2 (100) | 3 (100) | 19 (90.5) | 4 (100) | 3 (100) | 53 (82.8) |
| Positive | 7 (22.6) | 0 (0) | 0 (0) | 2 (9.5) | 0 (0) | 0 (0) | 9 (14.1) |
| EGFR | |||||||
| Negative | 26 (83.9) | 2 (100) | 2 (66.7) | 19 (90.5) | 3 (75) | 2 (66.7) | 54 (84.4) |
| Positive | 3 (9.7) | 0 (0) | 1 (33.3) | 2 (9.5) | 1 (25) | 1 (33.3) | 8 (12.5) |
| Subtype | |||||||
| Luminal A | 22 (71) | 2 (100) | 2 (66.7) | 16 (76.2) | 4 (100) | 3 (100) | 49 (76.6) |
| Luminal B | 6 (19.4) | 0 (0) | 1 (33.3) | 4 (19) | 0 (0) | 0 (0) | 11 (17.2) |
| HER2+ | 0 (0) | 0 (0) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 1 (1.6) |
| Triple-(basal like) | 1 (3.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download